

Aldehydes and Ketones
Aldehydes and ketones are the simplest and most important carbonyl compounds.
There are two systems of nomenclature of aldehydes and ketones.
Aldehydes:
Common names of aldehydes:
The common names of aldehydes are derived from the common names of the corresponding carboxylic acids in which the ending ‘-ic’ is replaced with ‘-aldehyde’of acid with the aldehyde. For example:
 IUPAC names of aldehydes:
IUPAC names of aldehydes:
In IUPAC system, the suffix ‘e’ of the alkane is replaced by the ‘al’.
For example:

Ketones
Common names of ketones:
The common names of ketones are derived by naming two alkyl or aryl groups bonded to the carbonyl group.
For example:
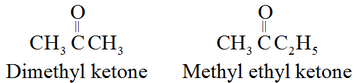
Alkyl phenyl ketones are usually named by adding the acyl group as a prefix to phenone. For example:
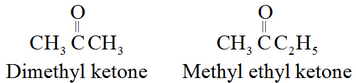
IUPAC name of ketones:
In IUPAC system, the suffix ‘e’ of the alkane is replaced by the ‘one’. For example:
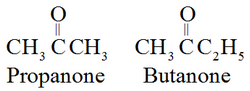
Structure of the Carbonyl Group
- The carbonyl carbon atom is sp2-hybridised and forms three sigma (σ) bonds. The fourth valence electron of carbon remains in its p-orbital and forms a π-bond with oxygen by overlap with p-orbital of an oxygen.
- In addition, the oxygen atom also has two non-bonding electron pairs. Thus, the carbonyl carbon and the three atoms attached to it lie in the same plane and the π-electron cloud is above and below this plane.

- The high polarity of the carbonyl group is explained on the basis of resonance involving a neutral (A) and a dipolar (B) structures.


