
Acids and Bases in the Laboratory:
-
Litmus Paper Test:
Acid converts blue litmus paper to red in color.
Bases convert red litmus paper to blue in color.
How do Acids and Bases React with Metals?
Acids react with metal:
Acids react with Metals and form salt and hydrogen gas.
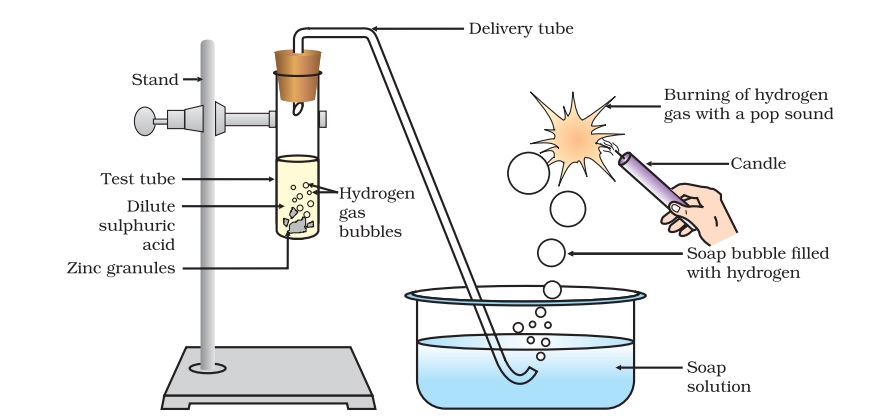
The metal in the above reactions displaces hydrogen from the acids. The metal combines with the remaining part of the acid and forms a compound called a salt.
Thus, the reaction of a metal with an acid can be summarized as –
Acid + Metal → Salt + Hydrogen gas
Bases react with metals:
Bases also react with metals and give hydrogen gas. But bases don’t react with all metals.
2NaOH + Zn →Na2ZnO2 + H
How do Acids and Bases React with each other?
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
The reaction between an acid and a base to give a salt and water is known as a neutralization reaction.
In general, a neutralization reaction can be written as –
Base + Acid → Salt + Water


really helpful i am searching for chemical properties and in every where just showing physical properties thanks for sharing this article….