
Crystal Lattices:
A crystalline solid consist of a large number of small units, called crystals, each of which possesses a definite geometric shape bounded by plane faces. The crystals of a given substance produced under a definite set of conditions are always of the same shape.
Unit Cells:
The smallest part of the crystal lattice is known as a unit cell. Look at the role of the unit cell in the crystal lattice.
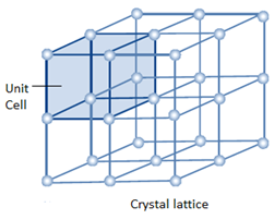
Bravais Lattices:
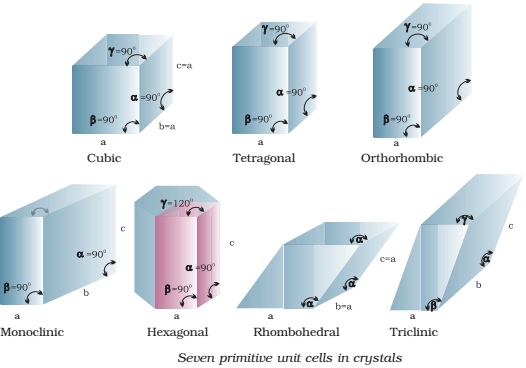
Bravais (1848) showed from geometrical considerations that there are only seven shapes in which unit cells can exist.
- Cubic
- Orthorhombic
- Rhombohedral
- Hexagonal
- Tetragonal
- Monoclinic
- Triclinic.
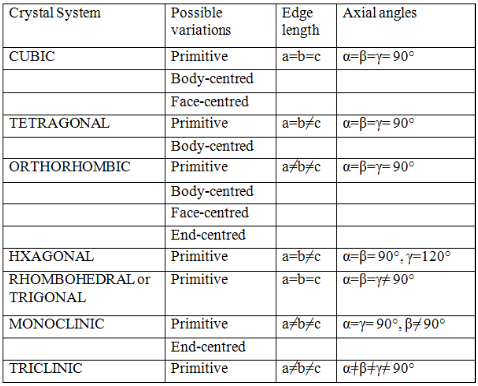
Moreover, he also showed that there are basically four types of unit cells depending on the manner in which they are arranged in a given shape.
- Primitive
- Body-Centered
- Face Centered
- End Centered.
He also went on to postulate that out of the possible twenty-eight unit cells (i.e. seven shapes ´ four types in each shape = 28 possible unit cells), only fourteen actually would exist. These he postulated based only on symmetry considerations. These fourteen unit cells that actually exist are called Bravais Lattices.

