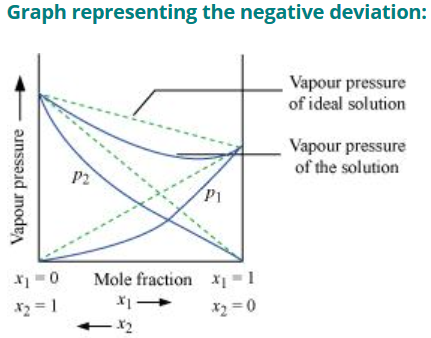
Positive Deviation from Roult’s Law
- The vapour pressure of solution formed by mixing two components is higher than predicted from Roult’s Law
- The new intermolecular interactions formed by mixing the component A and B (A‒B) are weaker than the intermolecular interactions of pure component (A‒A and A‒B)
- Example: mixture of ethanol and acetone, solution of carbon disulphide and acetone.
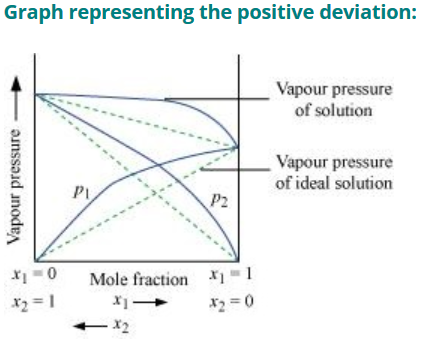
Negative Deviation from Roult’s Law
- The vapour pressure of solution formed by mixing two components is lower than predicted from Roult’s Law
- The new intermolecular interactions formed by mixing the component A and B (A-B) are stronger than the intermolecular interactions of pure component (A-A and A-B)
- Example: solution of phenol and aniline, chloroform and acetone.