
Electrochemistry
Electrochemistry is the study of the production of electricity from the energy released during a spontaneous chemical reaction and the use of electrical energy to bring about non-spontaneous chemical transformations.
Electrochemical cells:
A spontaneous chemical process is the one which can take place on its own and in such a process Gibb’s energy of the system decreases. It is this energy that gets converted into electrical energy. The reverse process is also possible in which we can make non-spontaneous processes occur by supplying external energy in the form of electrical energy. These interconversions are carried out in equipment’s called Electrochemical Cells.
Electrochemical Cells are of two types:
- Galvanic Cells
- Electrolytic Cells
Galvanic Cells
Cell energy is extracted from a spontaneous chemical process or reaction and it is converted to electric current. For example, Daniell Cell is a Galvanic Cell in which Zinc and Copper are used for the redox reaction to take place.
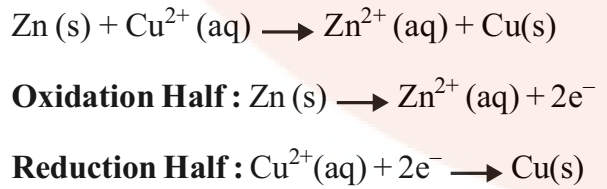
Zn is the reducing agent and Cu2+ is the oxidizing agent. The half cells are also known as Electrodes. The oxidation half is known as Anode and the reduction half is called Cathode. Electrons flow from anode to cathode in the external circuit. The anode is assigned negative polarity and cathode is assigned positive polarity. In Daniell Cell, Zn acts as the anode and Cu act as the cathode.
These reactions occur in two different portions of the Daniell cell. The reduction half-reaction occurs on the copper electrode while the oxidation half-reaction occurs on the zinc electrode. These two portions of the cell are also called half-cells or redox couples. The copper electrode may be called the reduction half-cell and the zinc electrode, the oxidation half-cell.
Electrolytic Cell
These electrodes are dipped in an electrolytic solution containing cations and anions. On supplying current the ions move towards electrodes of opposite polarity and simultaneous reduction and oxidation take place.
Preferential Discharge of ions: Where there are more than one cation or anion the process of discharge becomes competitive in nature. Discharge of any ion requires energy and in case of several ions being present, the discharge of that ion will take place first which requires the energy.

