
Group 15 Elements
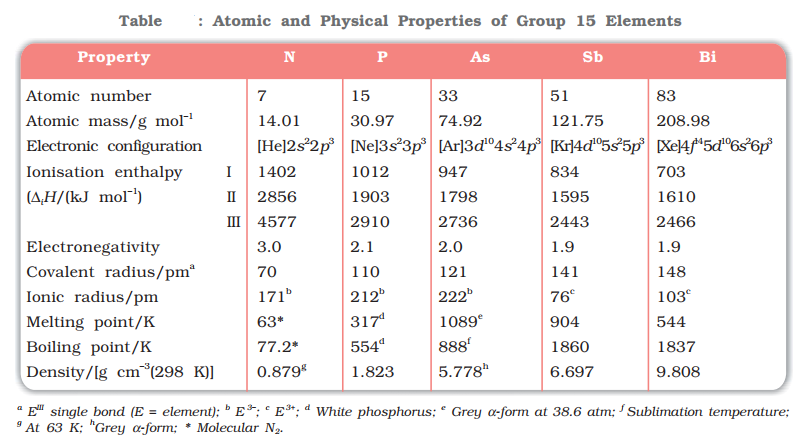
- Group 15 includes nitrogen, phosphorus, arsenic, antimony and bismuth.
- As we go down the group, there is a shift from non-metallic to metallic through metalloidic character.
- Nitrogen and phosphorus are non-metals, arsenic and antimony metalloids and bismuth is a typical metal.
Occurrence
Molecular nitrogen comprises 78% by volume of the atmosphere. In the earth’s crust, it occurs as sodium nitrate, NaNO3 (called Chile saltpetre) and potassium nitrate (Indian saltpetre). It is found in the form of proteins in plants and animals. Phosphoproteins are present in milk and eggs. Arsenic, antimony and bismuth are found mainly as sulphide minerals.
Electronic Configuration
- The valence shell electronic configuration of these elements is ns2np3.
- The s orbital in these elements is completely filled and p orbitals are half-filled, making their electronic configuration extra stable.

