
Integrated Rate Equations
The integrated rate equations are different for the reactions of different reaction orders. We shall determine these equations only for zero and first order chemical reactions.
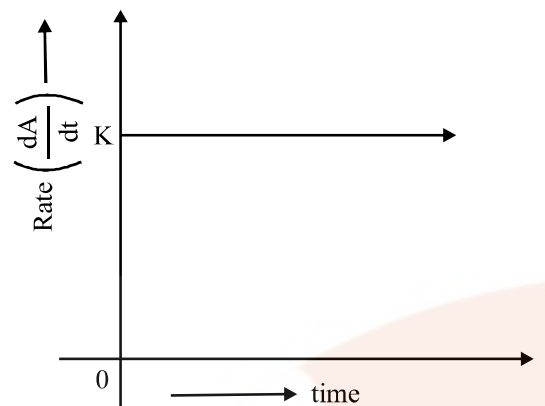
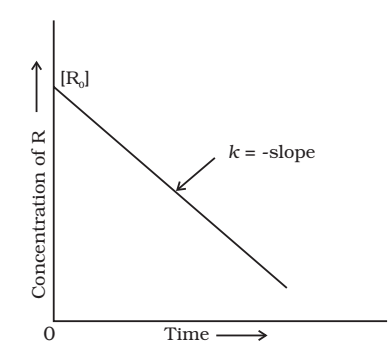
Zero Order Reaction
The rate of the reaction is proportional to zero power of the concentration of reactants.
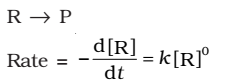
As any quantity raised to power zero is unity
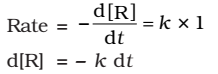
Integrating both sides
![]()
Where, I is the constant of integration.
At t = 0, the concentration of the reaction R = [R]0 , where [R]0 is initial concentration of the reaction.
Substituting in equation,
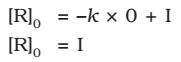
Substituting the value of I
![]()
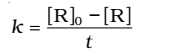
Example:
- The decomposition of gaseous ammonia on a hot platinum surface at high pressure.
![]()
![]()
- Thermal decomposition of HI on a gold surface.
First Order Reaction
The rate of the reaction is proportional to the first power of the concentration of the reactant R.
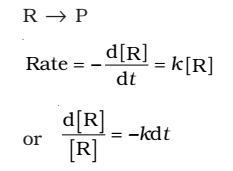
Integrating this equation, we get
![]()
Again, I is the constant of integration and its value can be determined easily.
When t= 0, R= [R]0, where [R]0 is the initial concentration of the reactant.
Therefore, equation can be written as
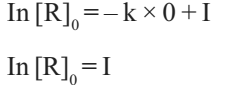
Substituting the value of I in equation
![]()
Rearranging this equation
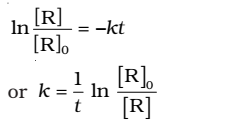
At time t1 from above eq.
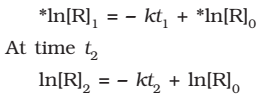
Where, [R]1 and [R]2 are the concentrations of the reactants at time t1 and t2 respectively.
Subtracting
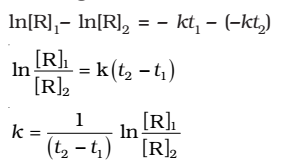
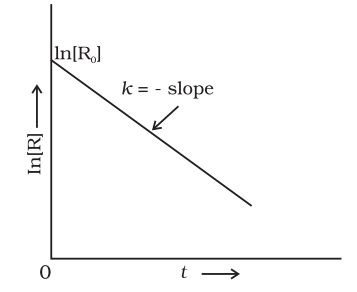
Comparing equation (2) with y = mx +c, if we plot In[R] against t, we get a straight line with slope = -k and intercept equal to ln[R]0
The first order rate equation (3) can also be written in the form
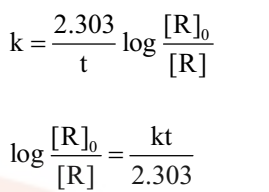
Example:
- Hydrogenation of ethane,
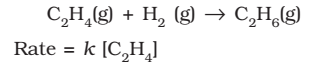
- Decomposition of N2O5 and N2O

