
Physical Properties of Carboxylic acids
- Carboxylic acids are higher boiling liquids than aldehydes, ketones and even alcohols of comparable molecular masses.
- Aliphatic carboxylic acids upto nine carbon atoms are colourless liquids at room temperature with unpleasant odours. The higher acids are wax like solids and are practically odourless due to their low volatility.
- The solubility decreases with increasing number of carbon atoms. Higher carboxylic acids are practically insoluble in water due to the increased hydrophobic interaction of hydrocarbon part.
- Benzoic acid, the simplest aromatic carboxylic acid is nearly insoluble in cold water.
- Carboxylic acids are also soluble in less polar organic solvents like benzene, ether, alcohol, chloroform, etc.
Chemical Reactions of Carboxylic acids
Reactions Involving Cleavage of O-H Bond
- Acidity (Reactions with metals and alkalies): Carboxylic acids dissociate in water to give resonance stabilized carboxylate anions and hydronium ion.
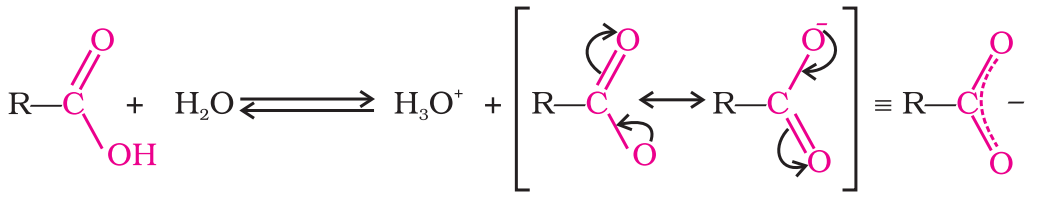
Reactions Involving Cleavage of C-OH Bond
- Formation of anhydride: Carboxylic acids on heating with mineral acids such as H2SO4 or with P2O5 give corresponding anhydride.

- Esterification: Carboxylic acids are esterified with alcohols or phenols in the presence of a mineral acid such as concentrated H2SO4 or HCl gas as a catalyst.
![]()
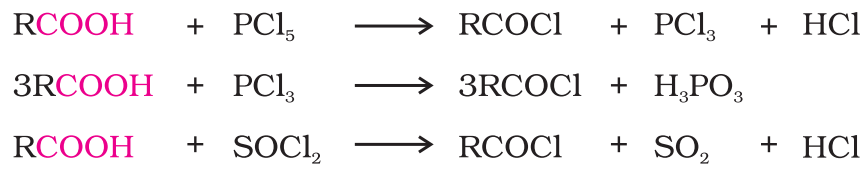
- Reactions with PCl5, PCl3 and SOCl2: The hydroxyl group of carboxylic acids, behaves like that of alcohols and is easily replaced by chlorine atom on treating with PCl5, PCl3 and SOCl2.
Thionyl chloride (SOCl2) is preferred because the other two products are gaseous and escape the reaction mixture making the purification of the products easier.
- Reaction with ammonia: Carboxylic acids react with ammonia to give ammonium salt which on further heating at high temperature give amides. For example:

Reactions Involving -COOH Group
- Reduction: Carboxylic acids are reduced to primary alcohols by lithium aluminium hydride or better with diborane.
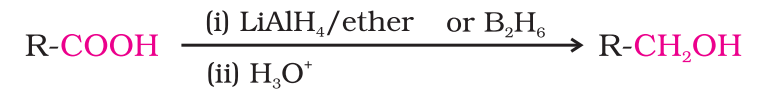
- Decarboxylation: Carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with sodalime (NaOH and CaO in the ratio of 3 : 1). The reaction is known as decarboxylation.

Substitution Reactions in the Hydrocarbon Part
- Halogenation: Carboxylic acids having an α-hydrogen are halogenated at the α-position on treatment with chlorine or bromine in the presence of small amount of red phosphorus to give α-halocarboxylic acids. The reaction is known as Hell-Volhard-Zelinsky reaction.

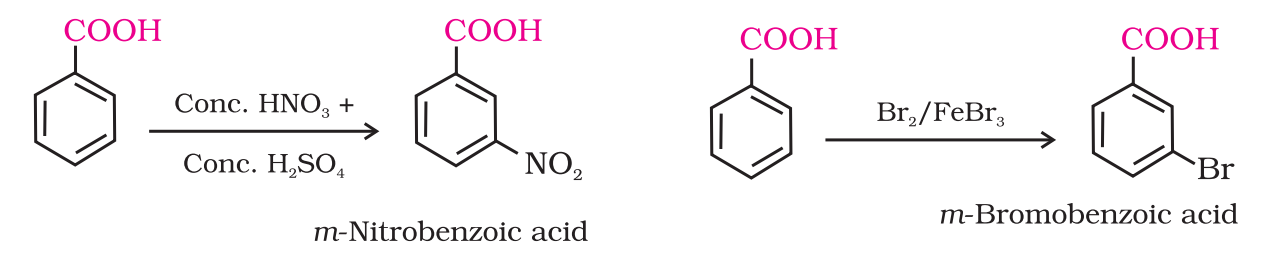
- Ring substitution: Aromatic carboxylic acids undergo electrophilic substitution reactions in which the carboxyl group acts as a deactivating and meta-directing group. They however, do not undergo Friedel-Crafts reaction.
Uses of Carboxylic Acids
- Methanoic acid is used in rubber, textile, dyeing, leather and electroplating industries.
- Ethanoic acid is used as solvent and as vinegar in food industry.
- Hexanedioic acid is used in the manufacture of nylon-6, 6.
- Esters of benzoic acid are used in perfumery. Sodium benzoate is used as a food preservative.
- Higher fatty acids are used for the manufacture of soaps and detergents.

