
Physical properties of Phenols
- Phenols are colourless liquids or crystalline solids but become coloured due to slow oxidation with air.
- Due to the presence of strong intermolecular hydrogen bonding, phenols have a higher boiling point than the corresponding hydrocarbon or aryl halides.
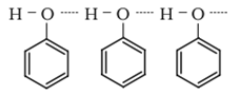
- Due to their ability to form hydrogen bonds with water, phenols are moderately soluble in H2O.
- The phenols are acidic in nature and stronger acids than alcohols. This is due to the fact that the sp2 hybridised carbon of phenol to which −OH is attached, is highly electronegative which causes a decrease in electron density on oxygen. This Increases the polarity of O−H bond and results in an increase in ionisatlon of phenols than that of alcohols.
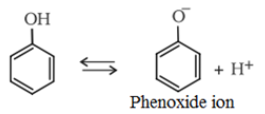
Moreover, the phenoxide ion so produced is stabilised by the delocalization of charge in phenol.
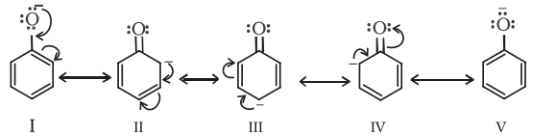
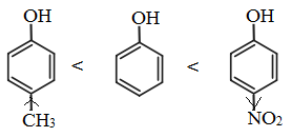
Note: The presence of electron withdrawing group like NO2 group, increases the acidic strength whereas the electron donating groups like an alkyl group decreases the acidic strength. Therefore, the acidic strength order is
Chemical properties of phenols
Electrophilic substitution reactions
The presence of OH group on benzene increases the electron density on the benzene ring making it more susceptible to attack by an electrophile. The reactions involving benzene ring are electrophilic substitution reaction. The presence of OH group makes the orthoand para carbon of benzene more electron rich than meta position. The OH group is called o ‒, p ‒ directing group.
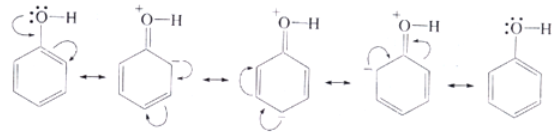
Reactions of phenol involving the cleavage of O–H bond
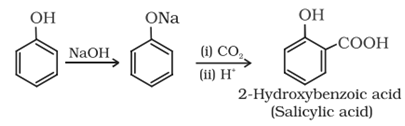
Kolbe’s reaction:
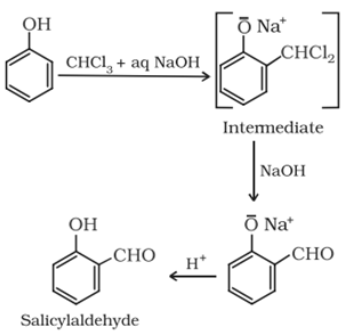
Reimer-Tiemann reaction:
Fries rearrangement:
Esters of phenol gives phenolic ketones on rearrangement in the presence of anhydrous AlCl3. This reaction is called fries rearrangement.
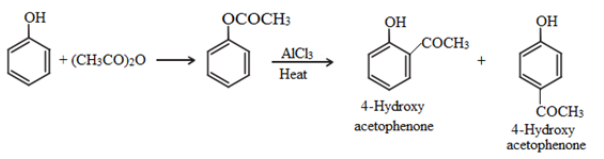
Acetylation:
Nitration:
Reaction with dilute HNO3 :
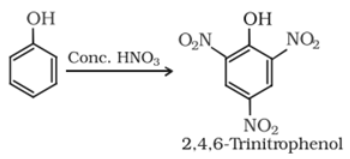
Reaction with conc. HNO3 :
Halogenation:
Bromination in solvents of low polarity like CS2 :
The reaction of phenol with bromine water:
Reactions of phenol involving cleavage of the C-O bond
Reaction with zinc dust
Reaction with ammonia:
Uses of phenol
- It is used as an antiseptic.
- It is used as a disinfectant in household cleaners.
- It is used in the preparation of resins, dyes, explosives, lubricants, pesticides, plastics, drugs, etc.








Very useful notes at higher secondary classes level.
Mr SUGEET SETHI
Faculty member Chemistry
NUCLEUS EDUCATION CENTER UJJAIN