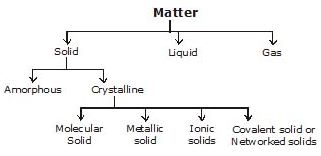
Classification of Crystalline Solid
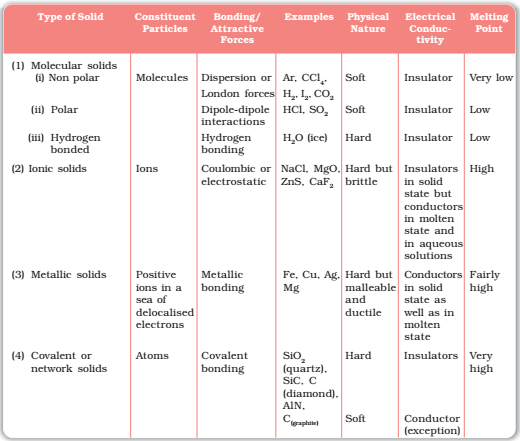
Molecular solids:
Those solids which consist of small molecules are called molecular solids.
1. Non – polar molecular solids:
- The solids which have zero dipole moment are called non-polar molecular solids.
- The molecules are held together by weak Vander Waal’s forces. Hence they are either gas or liquids at room temperature.
- They are a poor conductor of electricity due to their non-polar nature.
(electronegativity sequence (F > O > N ≈ Cl > Br > S > C H)
2. Polar molecular solids:
- Those solids which have non-zero dipole moments are called polar molecular solids.
- Polar molecular solids have dipole-dipole interaction, which is slightly stronger than Vander Waal’s force and hence they have larger melting and boiling points than the non – polar molecular solids.
- They are generally liquids or gases at room temperature.
Example: Solid CO2, Solid NH3
3. Hydrogen-bonded molecular solids:
- Those molecular solids which are bonded to each other by hydrogen bonds are called hydrogen-bonded molecular solids.
- They are non-conductor of electricity.
- Generally, they are liquid at room temperature or soft solids.
Example: Ice
IONIC SOLIDS:
All those solids whose constituent particles are ions are called ionic solids. Such solids are formed by the three-dimensional arrangements of cations and anions bound by strong coulombic (electrostatic) forces.
- These solids are hard and brittle in nature.
- They have high melting and boiling points.
- Since the ions are not free to move about, they are electrical insulators in the solid state. However, in the molten state or when dissolved in water, the ions become free to move about and they conduct electricity.
Example: NaCl, CsBr, AgBr, CsCl
METALLIC SOLIDS:
- All those solids which are bonded by metallic bonds are called metallic solids.
- Inner core electrons are immobile
- Metallic solids show a great electrical conductivity due to the availability of a large number of free electrons whose movements constitute an electric current.
- Metallic solids are malleable and ductile.
- Metallic solids have lustre.
- Metallic solids have good thermal conductivity.
COVALENT SOLIDS OR NETWORKED SOLIDS:
Whenever an electric field is applied, electrons move between the layers. Graphite is a good conductor of electricity due to the availability of free electrons. Networked solids are hard and brittle. Carbon-carbon bond has got a partial double bond character in graphite. Two layers of graphite are attached to each other by weak Vander Waal force. Graphite can be used as a lubricant at high temperatures.