Refining
The process of purifying the crude metals is called refining. Some methods of refining are as follow:
- Distillation: This is very useful for low boiling metals like zinc and mercury. The impure metals are evaporated to obtain the pure metals as distillate.
- Liquation: In this method, a low melting metal like tin can be made to flow on a sloping surface. In this way, it is separated from higher melting impurities.
- Electrolytic refining: In this method, the impure metal is made to act as anode a strip of some metal in pure form is used as a cathode. They are put in a suitable electrolytic bath containing a soluble salt of some metals the more basic metal remains in the solution and the less basic ones go to the anode mud.
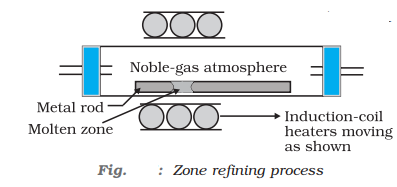
- Zone refining: In this method, a circular mobile heater is fixed at one end of a rod or the impure metal, as heater more forward, the pure metal crystallises out of the melt and impurities pass on into the adjacent molten zone.
- Vapour phase refining: The crude metals is free from impurities by first converting it into a suitable volatile compound by heating it with the specific reagent at a lower temperature and then decomposing the volatile compound at some higher temperature to give the pure metal.
So, the two requirements are:
- the metal should form a volatile compound with an available reagent,
- the volatile compound should be easily decomposable so that the recovery is easy.
Following examples will illustrate this technique:
- Mond Process: In this process, nickel is heated in a stream of carbon monoxide forming a volatile complex, nickel tetracarbonyl:
![]()
![]()
- Van Arkel method: This method is very useful for removing all the oxygen and nitrogen present in the form of impurity in certain metals like Zr and Ti. The crude metal is heated in an evacuated vessel with iodine. The metal iodide being more covalent, volatilizes:
![]()
![]()
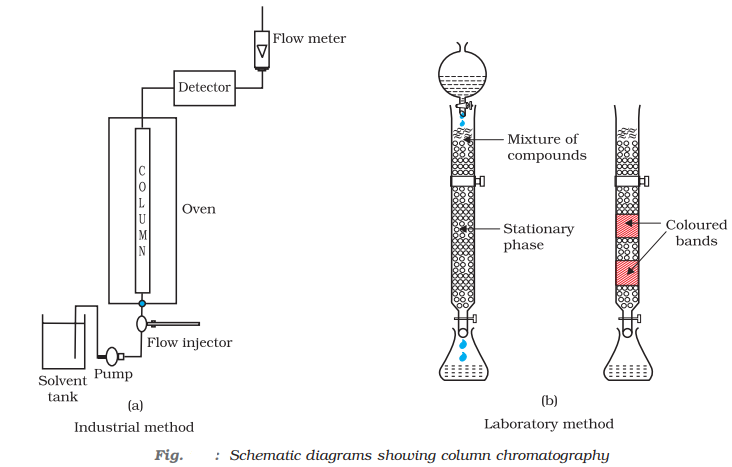
- Chromatographic method: The method is based upon the principle that the different components of a mixture are adsorbed to different extents of an adsorbent.
For example, in column chromatography, different components of the mixture are adsorbed to different extents depending on their polarity.