
Sulphur (S)

Sulphur exhibits allotropy, two important allotropes of which are:
- Yellow Rhombic (α – sulphur)
- Monoclinic (β- sulphur)

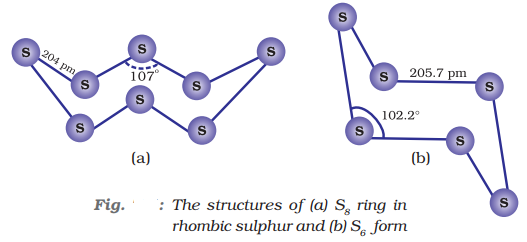
At 369 K both forms are stable. S8 puckered shape in both forms and has crown shape.


Sulphur Dioxide (SO2)
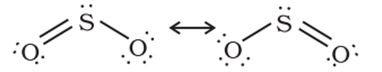
The molecule of SO2 is angular. It is a resonance hybrid of the two canonical forms:
Preparation of Sulphur dioxide:
Sulphur dioxide is formed together with a little (6-8%) sulphur trioxide when sulphur is burnt in air or oxygen:
![]()
In the laboratory, it is readily generated by treating a sulphite with dilute sulphuric acid.
![]()
Properties of Sulphur dioxide:
- A colourless gas with burning sulphur smell.
- It is heavier than air and is highly soluble in water.
- Acidic Nature: Acidic oxide and thus dissolve in water forming sulphurous acid.
![]()
- Addition Reaction
![]()
- SO2 is a reducing agent.
For example:
Uses of Sulphur dioxide:
- SO2 is used in refining petroleum and sugar.
- It is used in bleaching wool and silk.
- It is also used as a disinfectant and preservative.
- It is used in the manufacture of sulphuric acid, sodium hydrogen sulphite and calcium hydrogen sulphite.
Oxoacids of Sulphur
Sulphur forms a variety of oxoacids. All oxoacids of sulphur are dibasic.
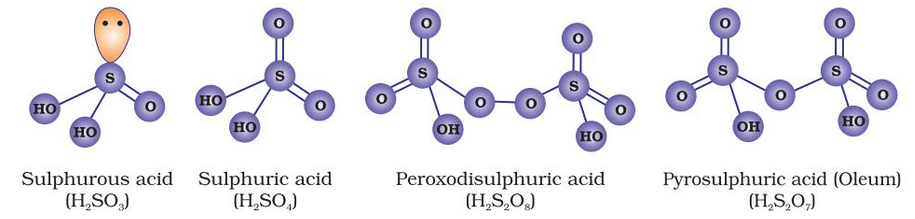
Sulphur forms a number of oxoacids such as H2SO3, H2S2O3, H2S2O4, H2S2O5, H2SxO6 (x = 2 to 5), H2SO4, H2S2O7, H2SO5, H2S2O8. Some of these acids are unstable and cannot be isolated. They are known in aqueous solution or in the form of their salts. Structures of some important oxoacids are shown in the figure given below
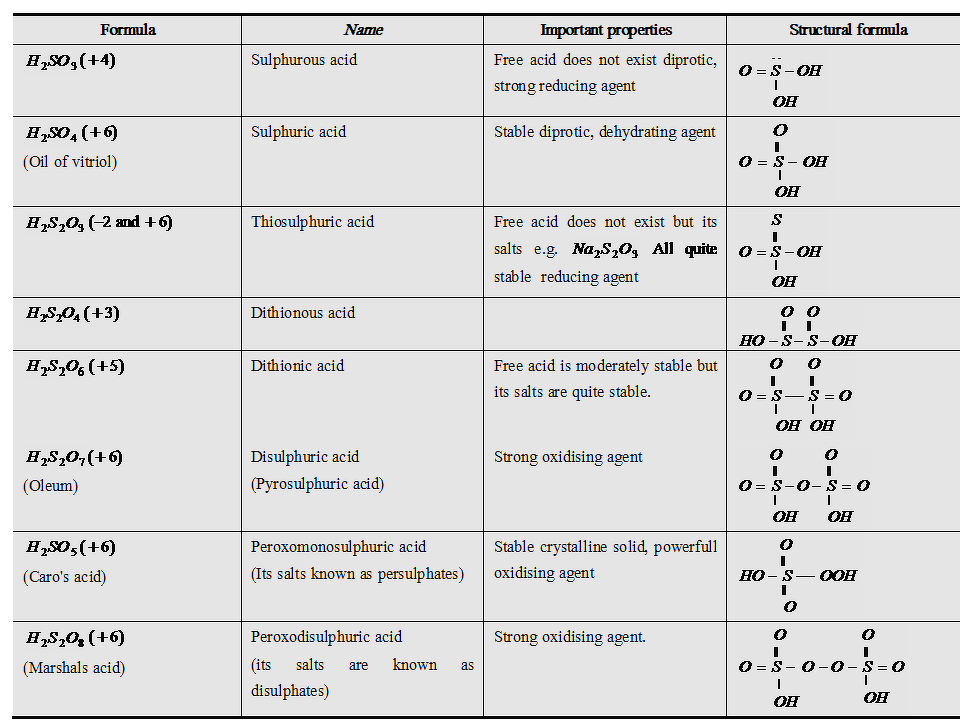
Sulphuric acid (H2SO4)
Preparation of Sulphuric acid:
Sulphuric acid is manufactured by the Contact Process as follows:
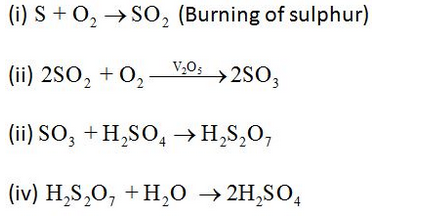
Properties of Sulphuric acid:
- Sulphuric acid is a colourless, dense oily liquid.
- It is dibasic acid or diprotic acid.
- It is a strong dehydrating agent.
- It is a moderately strong oxidizing agent.
Uses of Sulphuric acid:
- H2SO4 is used in the manufacture of fertilisers.
- It is also used in petroleum refining, manufacture of pigments, detergents, metallurgical application and storage batteries.
Industrial process of manufacturing of Sulphuric acid
Sulphuric acid is one of the most important industrial chemicals worldwide. Sulphuric acid is manufactured by the Contact Process which involves three steps:
- burning of sulphur or sulphide ores in the air to generate SO2.
- conversion of SO2 to SO3 by the reaction with oxygen in the presence of a catalyst (V2O5), and
- absorption of SO3 in H2SO4 to give Oleum (H2S2O7)
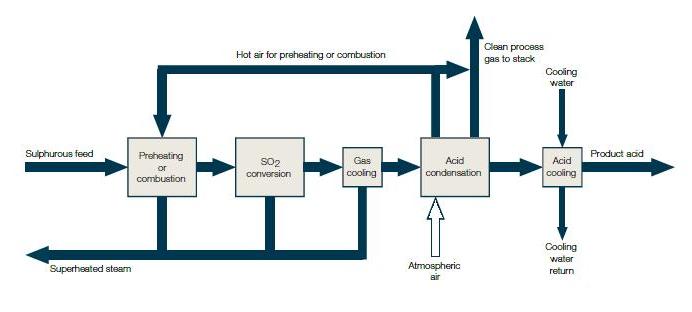
A flow diagram for the manufacture of sulphuric acid is shown in (Fig). The SO2 produced is purified by removing dust and other impurities such as arsenic compounds.
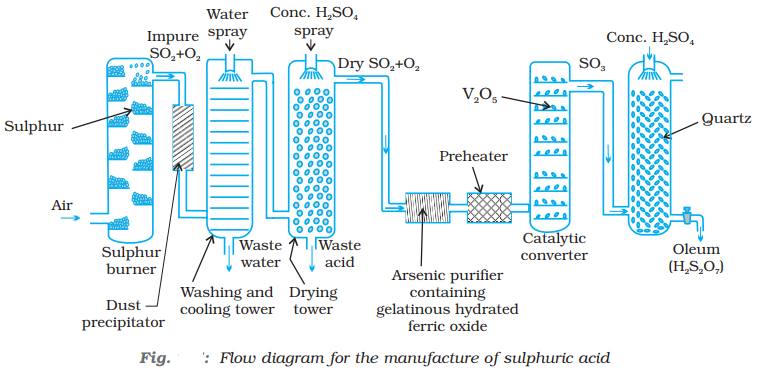
Step 1: Preparation of sulphur dioxide:
SO2 is prepared by burning sulphur in the presence of excess air so that the product combines with oxygen which is helpful for the next stage.
![]()
Step 2: Preparation of sulphur trioxide:
Sulphur trioxide is formed when sulphur dioxide reacts with oxygen in a ratio of 1:1 at a temperature of 400°C – 450°C and a pressure of 1-2 atm in the presence of N2O5 as a catalyst. This reaction is reversible in nature.
![]()
Step 3: Preparation of concentrated sulphuric acid:
The sulphur trioxide formed is first made to react with concentrated sulphuric acid. Sulphur trioxide cannot be dissolved in water directly as it leads to the formation of fog. The product obtained after this reaction is known as oleum. The oleum obtained is then dissolved in water to obtain concentrated sulphuric acid.
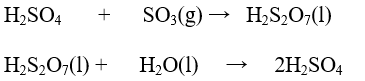
Uses of sulphuric acids:
In Industries:
- Up to 50 % of this liquid manufactured is used in the production of phosphoric acid which is in turn used to make phosphate fertilizers.
- It is used in the manufacturing of metals such as copper, zinc etc.
- 5% of the produced acid is used in the making of fibres.
In Domestic Environments:
- Used in acidic drain cleaners.
- Due to its strong dehydrating property, it can be used to remove tissue paper.
As a Catalyst:
- Used as a catalyst in the manufacturing process of nylon.
- Used in the Manheim process in the manufacturing of HCl.
- Used in petroleum refining.

