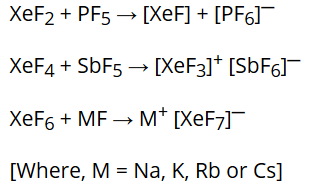Xenon-fluorine compounds
Xenon forms three binary fluorides, XeF2, XeF4 and XeF6 .
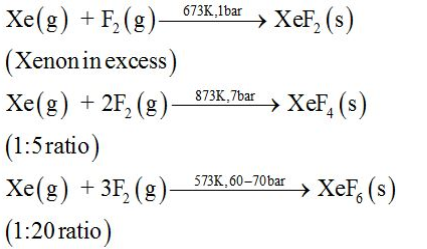
Preparation of Xenon-fluorine compounds:
Properties of Xenon-fluorine compounds:
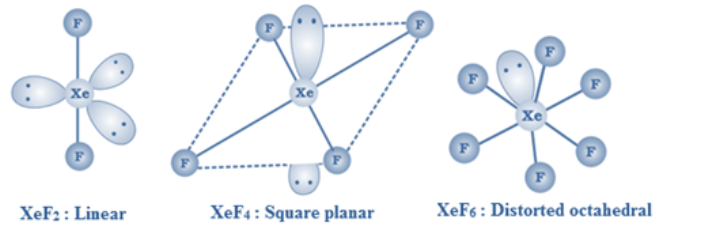
XeF2 is linear, XeF4 is square planar and XeF6 is distorted octahedral.
XeF2, XeF4 and XeF6 are colourless crystalline solids
They are readily hydrolysed.
![]()
They react with fluoride ion acceptors to form cationic species and fluoride ion donors to form fluoroanions.