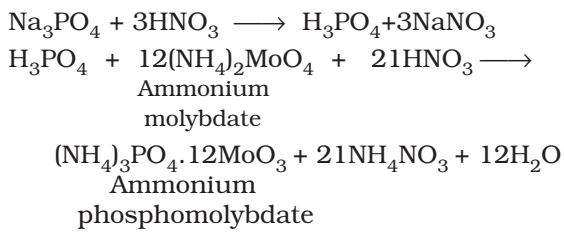Detection of Carbon and hydrogen: Put Copper Oxide Test
Carbon and hydrogen are detected by heating the compound with copper (II) oxide. Carbon present in the compound is oxidized to carbon dioxide (tested with lime-water, which develops turbidity) and hydrogen to water (tested with anhydrous copper sulphate, which turns blue).
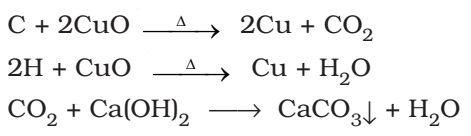
Detection of Other elements
Lassaigne’s test:
Nitrogen, sulphur, halogens and phosphorus present in an organic compound are detected by ‘Lassaigne’s test’. Covalent compounds are converted into ionic form by fusing the compound with sodium metal. Following reaction occurs:
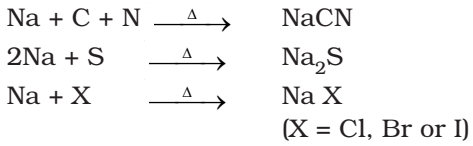
Test for Nitrogen
- The substance is heated strongly with sodium metal.
![]()
- The water extract of the fused mass is boiled with ferrous sulphate solution
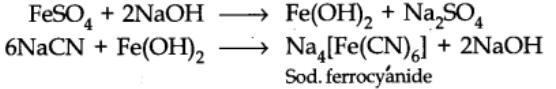
- To the cooled solution is then added a little ferric chloride solution and excess of conc. Hydrochloric acid.

The formation of Prussian blue or green colour confirms the presence of nitrogen.
Test for sulphur:
The sodium fusion extract is acidified with acetic acid and lead acetate is added to it. A black precipitate of lead sulphide indicated the presence of sulphur.
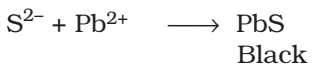
Test for Halogens:
The sodium fusion extract is acidified with nitric acid and then treated with silver nitrate. A white precipitate, soluble in ammonium hydroxide shows the presence of chlorine, a yellowish ppt. sparingly soluble in ammonium hydroxide shows the presence of bromine, a yellowish ppt. insoluble in ammonium hydroxide shows the presence of iodine.
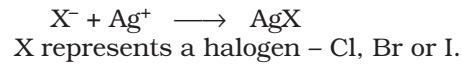
Test of Phosphorous:
The compound is heated with an oxidising agent (sodium peroxide). The phosphorous present in the compound is oxidised to phosphate.
The solution is boiled with HN03 and treated with ammonium molybdate. A yellow colored ppt. indicates the presence of phosphorous.