
Adsorption Isotherms
The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as adsorption isotherm.
A mathematical equation which describes the relationship between pressure (p) of the gaseous adsorbate and the extent of adsorption at any fixed temperature is called adsorption isotherms.
Thus, if x g of an adsorbate is adsorbed on m g of the adsorbent, then
Extent of adsorption = x/m
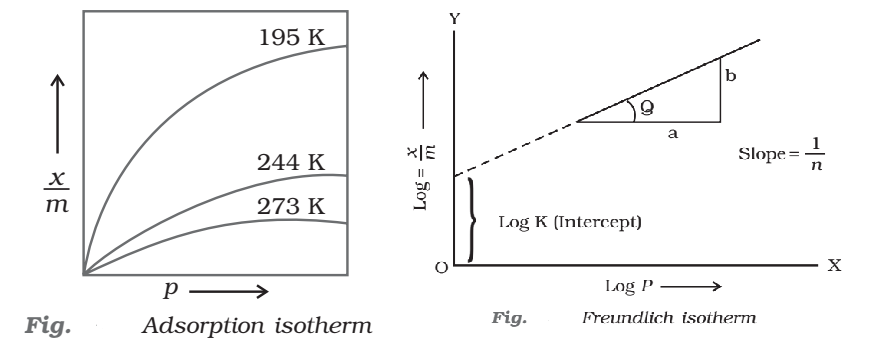
Freundlich adsorption isotherm: Freundlich adsorption isotherm is obeyed by the adsorptions where the adsorbate forms a monomolecular layer on the surface of the adsorbent.
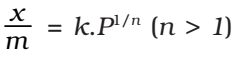
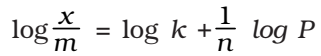
where, x is the weight of the gas adsorbed by m gm of the adsorbent at a pressure P, thus x/m represents the amount of gas adsorbed by the adsorbent per gm (unit mass), k and n are constant at a particular temperature and for a particular adsorbent and adsorbate (gas), n is always greater than one, indicating that the amount of the gas adsorbed does not increase as rapidly as the pressure.
At low pressure, the extent of adsorption varies linearly with pressure
![]()
At high pressure, it becomes independent of pressure
![]()
At moderate pressure x/m depends upon pressure raised to powers
![]()

