Amines
Amines are the organic compounds derived from ammonia (NH3) by replacing its one or more hydrogen atoms by alkyl or aryl group.
For example:
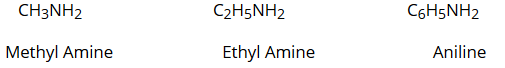
Structure of Amines
Nitrogen atom of amines carries an unshared pair of electrons and is sp3 hybridised with pyramidal shape. Due to the presence of unshared pair of electrons, the angle C–N–E, (where E is C or H) is less than 109.5° ; for instance, it is 108o in case of trimethylamine.

Classification of Amines
On the basis of number of hydrogen atoms replaced in NH3 molecule, amines are categorized into three types:
1° amine:
One hydrogen atom of NH3 is replaced by an alkyl or aryl group. For example: CH3NH2 (Methyl Amine)
2° amine:
Two hydrogen atoms of NH3 are replaced by alkyl or aryl groups. For example: CH3NHCH3 (Dimethyl Amine)
3° amine:
All three hydrogen atoms of NH3 are replaced by alkyl or aryl groups. For example:
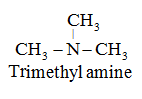
Nomenclature of Amines
Common naming system:
- Aliphatic amines are named by prefixing an alkyl group to a mine, i.e., alkylamine. For example:
- Secondary and tertiary amines, having two or more similar groups are named by adding prefix ‘di’ or ‘tri’ before the name of alkyl group. For example:
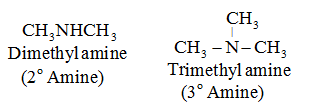
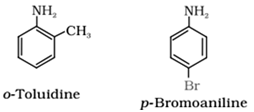
- Aromatic amines are named as derivatives of the parent member, aniline (C6H5NH2). For example:
IUPAC naming system:
- Aliphatic or aromatic amines are named by replacing ‘e’ in the end of the parent hydrocarbon by ‘amine’. For example:

- Amines containing more than one amino groups at different positions in the parent chain, are named by specifying numbers to the carbon atoms bearing –NH2 groups along with attaching a suitable prefix such as di, tri, etc. to the amine. The ending ‘e’ of the hydrocarbon is retained. For example:
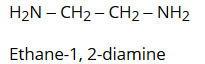
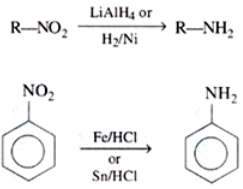
Preparation of Amines
Preparation of Amines By reduction of nitro (RNO2) compounds:
Preparation of Amines By reduction of nitriles (RCN):

Preparation of Amines By ammonolysis of alkyl halides:
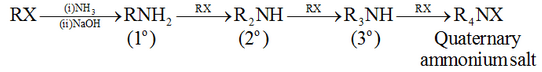
Preparation of Amines Hofmann bromamide reaction:
![]()
This method gives amine with one carbon less than the parent amide.
Preparation of Amines Gabriel phthalimide synthesis:
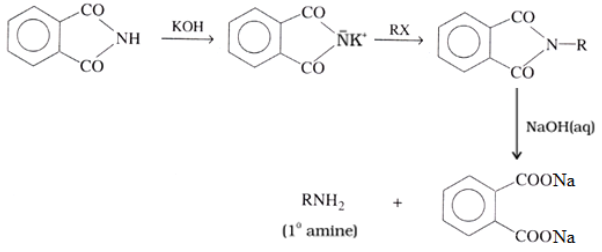
Physical Properties of Amines
- The lower aliphatic amines are gases with fishy smell.
- Primary amines with three or more carbon atoms are liquid and higher members are all solids.
- Lower amines are soluble in water as they can form hydrogen bonds with water, however the solubility decreases with increase in hydrophobic alkyl group.
- Amines have a higher boiling point than the hydrocarbon of comparable molecular mass. This is due to their ability to associate via intermolecular hydrogen bonding.
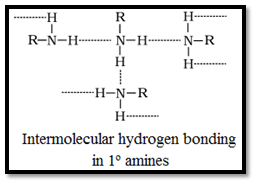
- Boiling points order of various isomeric amines is: 1o > 2o > 3o
Chemical Properties of Amines
Basic Character of amines:
Amines, being basic in nature, react with acids to form salts. Amines act as Lewis bases due to the presence of lone pair of electrons on the nitrogen atom. Basic character of amines can be better expressed in terms of their Kb and pKb values.

All aliphatic amines are strong bases than NH3 while aromatic amines are weaker bases than NH3 due to the electron withdrawing nature of the aryl group.
Factors affect the basicity of aliphatic amines are:
• Inductive effect
• Solvation effect
• Steric hindrance
Considering all the above factors the basic strength of methyl substituted amines and ethyl substituted amines in aqueous medium follows the order:
Alkylation of Amine:
Alkylation of 1o amine generates 2o amine, 3o amine and finally the quaternary salts.
![]()
Acylation of Amine:
Reaction with acid chlorides, anhydrides and esters by nucleophilic substitution reaction is known as acylation. The reaction is proceeded by the replacement of hydrogen atom of –NH2 or >N–H group by the acyl group (RCOX).
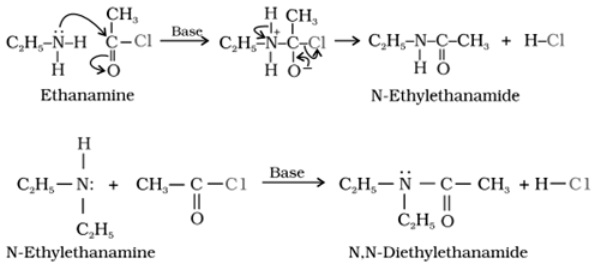
Tertiary (3o) amine cannot be acylated as there is no H bonded to nitrogen.
Benzoylation of Amine:
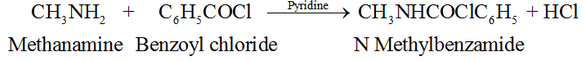
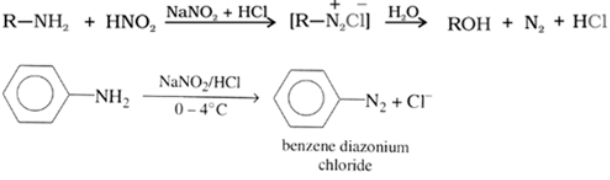
Reaction with nitrous acid, HNO2:
Three classes of amines react differently with nitrous acid.
Reaction of 10 amines:
Reaction of 20 amines:
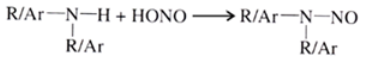
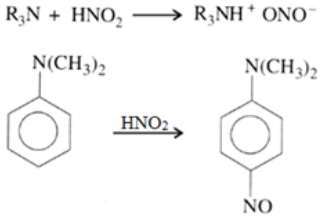
Reaction of 30 amines:
Carbylamine reaction:
Primary amine (both aliphatic and aromatic) reacts with CHCl3/KOH to form isocyanides (carbylamines) with unpleasant smell.
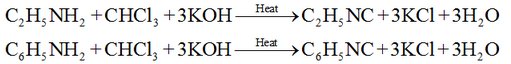
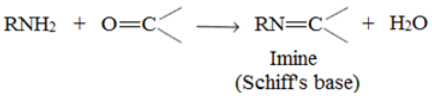
Reaction of Amine with aldehyde and ketones:
1o amines react with aldehydes and ketones to form Schiff’s bases.
Electrophilic substitution reactions:
Aniline, due to high electron density at ortho and para-positions, is ortho and para directing towards electrophilic substitution reaction.
Some of the electrophilic substitution reactions of aniline are described below:
Bromination of Amines:

To prepare monosubstituted aniline derivative, first acetylation of aniline is carried out with acetic anhydride followed by the desired substitution of the substituted amide which is then hydrolysed to obtain the monosubstituted amine.
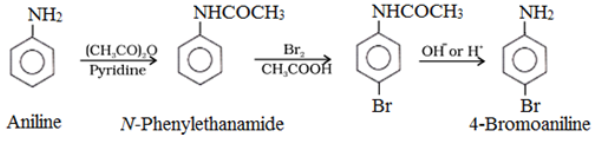
Nitration of Amines:

To get p-nitro derivative as major product, acetylation of aniline is carried out with acetic anhydride. Then desired substitution of anilide is carried out followed by hydrolysis of the substituted anilide to the substituted amine.
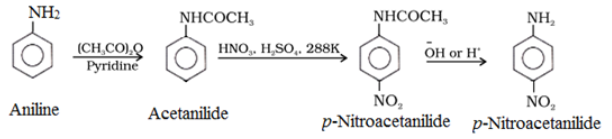
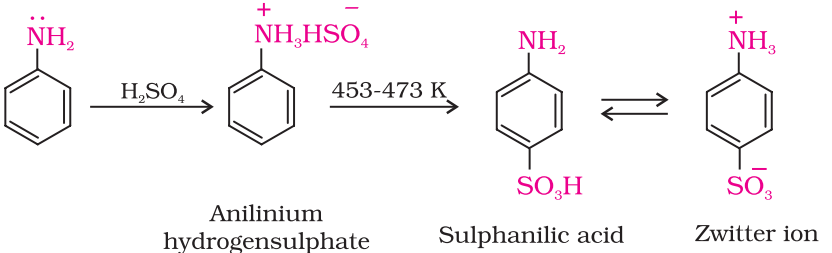
Sulphonation of Amines:
Aniline reacts with concentrated sulphuric acid to form anilinium hydrogensulphate which on heating with sulphuric acid at 453-473K produces p-aminobenzene sulphonic acid, commonly known as sulphanilic acid, as the major product.