
Batteries:
When Galvanic cells are connected in series to obtain a higher voltage the arrangement is called Battery.
Primary Batteries:
Primary cells are those which can be used so long the active materials are present. Once they get consumed the cell will stop functioning and cannot be re-used. Example Dry Cell or Leclanche cell and Mercury cell.
Dry Batteries
- Anode: Zn container
- Cathode: Carbon (graphite) rod surrounded by powdered MnO2 and carbon.
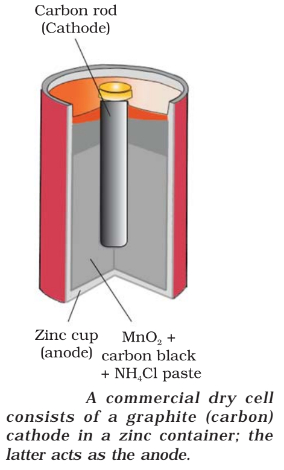
- Electrolyte: NH4Cl and ZnCl2
- Reaction:

The standard potential of this cell is 1.5 V and it falls as the cell gets discharged continuously and once used it cannot be recharged.
Mercury cells
These are used in small equipment like watches, hearing aids.
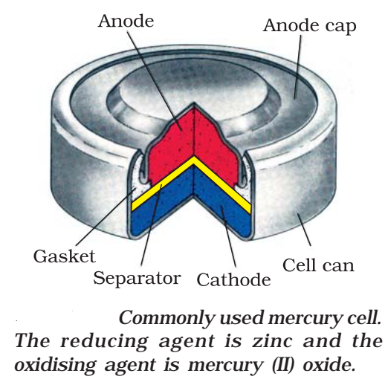
- Anode: Zn-Hg Amalgam
- Cathode: Paste of HgO and Carbon
- Electrolyte: Paste of KOH and ZnO
- Reaction:

The cell potential is approx. 1.35V and remains constant during its life.
Secondary Batteries:
Secondary cells are those which can be recharged again and again for multiple uses. e.g. lead storage battery and Ni-Cd battery.
Lead Storage Battery
- Anode: Lead (Pb)
- Cathode: Grid of lead packed with lead oxide (PbO2)
- Electrolyte: 38% solution of H2SO4

- Discharging Reaction

To recharge the cell, it is connected with a cell of higher potential and this cell behaves as an electrolytic cell and the reactions are reversed. Pb(s) and PbO2(s) are regenerated at the respective electrodes. These cells deliver an almost consistent voltage.


