Calorimetry means the measurement of heat. When a body at higher temperature is brought into contact with another body at a lower temperature, the heat lost by the hot body is equal to the heat gained by the colder body, provided no heat is allowed to escape to the surroundings. A device in which heat measurement can be made is called a calorimeter.
Heat gained = Heat lost
Change of State
Matter exists in three states: solid, liquid and gas. Any state of a substance can be changed into another by heating or cooling it. The transition of a substance from one side to another is called a change of state.
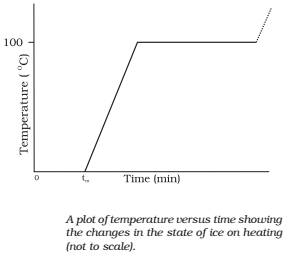
The common changes of states are as follows
- Melting of a solid:
- Vaporization of a liquid:
- Condensation of vapour:
- Freezing of a liquid:
Effect of pressure on the boiling point of a liquid:
The temperature at which the liquid and the vapour states of the substance coexist is called its boiling point.
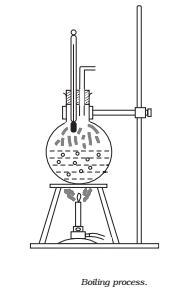
Let us do the following actions to understand the process of boiling of water.
Take a round-bottom flask, more than half filled with water. Keep it over a burner and fix a thermometer and steam outlet through the cork of the flask (Fig.)
- As water gets heated in the flask, note first that the air, which was dissolved in the water, will come out as small bubbles.
- Later, bubbles of steam will form at the bottom but as they rise to the cooler water near the top, they condense and disappear.
- Finally, as the temperature of the entire mass of the water reaches 100 °C, bubbles of steam reach the surface and boiling is said to occur. The steam in the flask may not be visible but as it comes out of the flask, it condenses as tiny droplets of water, giving a foggy appearance.
- If now the steam outlet is closed for a few seconds to increase the pressure in the flask, you will notice that boiling stops. More heat would be required to raise the temperature (depending on the increase in pressure) before boiling begins again. Thus boiling point increases with increase in pressure.
- Let us now remove the burner. Allow water to cool to about 80 °C. Remove the thermometer and steam outlet. Close the flask with the airtight cork. Keep the flask turned upside down on the stand. Pour ice-cold water on the flask. Water vapours in the flask condense reducing the pressure on the water surface inside the flask.
- Water begins to boil again, now at a lower temperature. Thus boiling point decreases with decrease in pressure
- The change from solid state to vapour state without passing through the liquid state is called sublimation, and the substance is said to sublime.
- Dry ice (solid CO2) sublimes, so also iodine. During the sublimation process, both the solid and vapour states of a substance coexist in thermal equilibrium.

