
Two types of detergents are used as cleansing agents. These are soaps and synthetic detergents.
Soap
Soaps are sodium or potassium salts of higher fatty acids (containing 15-18 carbon atoms) e.g., stearic acid, oleic acid and palmitic acid.
Saponification:
The process of producing soap by heating fat (glyceryl ester ~fatty acid) with aqueous sodium hydroxide solution, is termed as Saponification.
For example:

Soaps are not useful in hard water:
Hard water contains calcium and magnesium ions. The sodium salts present in soaps are converted to their corresponding calcium and magnesium salts which are precipitated as scum.
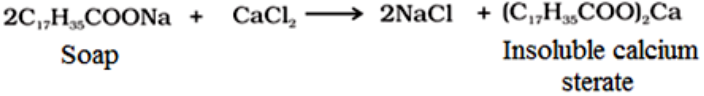
The insoluble scum sticks on the clothes hence reduce the cleaning capacity of soap.
Synthetic Detergents:
Detergents are sodium salt of a long-chain linear alkyl benzene sulphonic acid. These are manufactured chemically from materials other than animal fats.
These can be used both in soft and hard water as they give foam even in hard water.
Detergents are three types of detergents:
Anionic detergents:
These are sodium salts of sulphonated long chain alcohols or hydrocarbons. In anionic detergents, the anionic part of the molecule is involved in the cleansing action. For example: alkyl benzene sulphonates are obtained by neutralising alkyl benzene sulphonic acids with alkali.
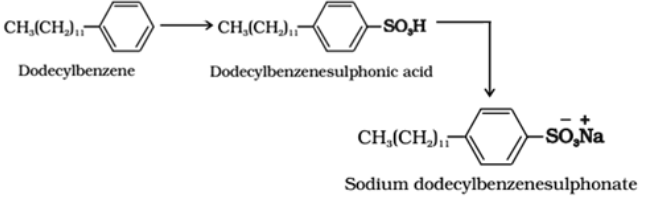
Use: They are also used in toothpaste.
Cationic detergents:
These detergents are quarternary ammonium salts of amines with acetates, chlorides or bromides as anions.
Cationic part possesses a long hydrocarbon chain and a positive charge on the nitrogen atom. For example: Cetyltrimethylammonium bromide is a popular cationic detergent used in hair conditioners.
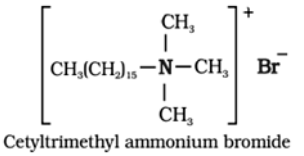
Use: They are used as germicides.
Non-ionic detergents:
These are the detergents that do not contain any ion in their constitution. For example: Esters of high molecular mass formed by reaction of polyethylene glycol and stearic acid are non-ionic detergents.
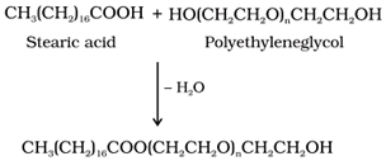
Use: They are used in liquid dishwashing detergents.

