
Expressing Concentration of Solutions
The composition of a solution is defined in terms of concentration. There are several ways to define the concentration of the solution as follows:
Mass percentage (w/w):
The mass percentage of a component of a solution is defined as:
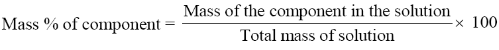
Volume percentage (v/v):
The volume percentage is defined as:
![]()
Mass by volume percentage (w/v):
Another unit which is commonly used in medicine and pharmacy is mass by volume percentage. It is the mass of solute dissolved in 100 mL of the solution.
![]()
Part per million (ppm):
When a solute is present in trace quantities, it is convenient to express concentration in parts per million (ppm).
![]()
Mole fraction:
A commonly used symbol for mole fraction is x and subscript used on the right-hand side of x denotes the component.
![]()
Molarity:
Molarity (M) is defined as a number of moles of solute dissolved in one litre (or one cubic decimeter) of the solution,
![]()
Molality:
Molality (m) is defined as the number of moles of the solute per kilogram (kg) of the solvent and is expressed as:
![]()

