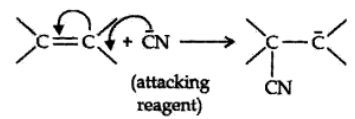
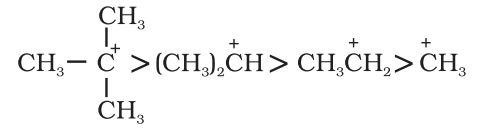The electrom’eric effect refers to the polarity produced in a multiple bonded compound when it is attacked by a reagent when a double or a triple bond is exposed to an attack by an electrophile E+ (a reagent) the two π electrons which from the π bond are completely transferred to one atom or the other. The electromeric effect is represented as:
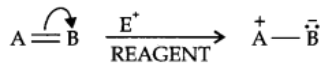
The curved arrow shows the displacement of the electron pair. The atom A has lost its share in the electron pair and B has gained this share. Therefore, A acquires a positive charge and B a negative charge.
There are two distinct types of electromeric effect.
Positive Electromeric Effect (+E effect):
In this effect the π electron of the multiple bond are transferred to that atom to which the reagent gets attached.
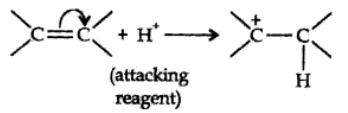
Negative Electromeric Effect (-E effect):
In this effect the π electron of the multiple bond are shifted to that atom to which the attacking reagent does not gets attached.
Hyperconjugation or No Bond Resonance
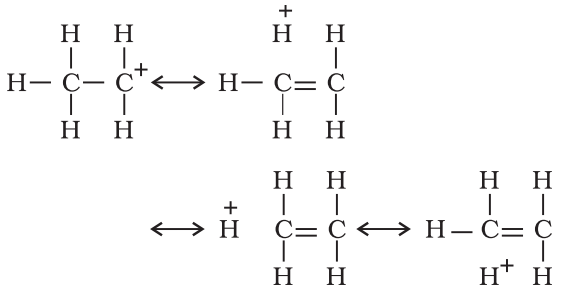
When the alkyl group is attached to an unsaturated system such as —CH=CH2 group the order of inductive effect gets reversed. The behavior can be explained by hyperconjugation effect.
Such structures are arrived at by shifting the bonding electrons from an adjacent C —H bond to the electron deficient carbon. In this way, the positive charge originally on carbon is dispersed to the hydrogen.
This way of electron release by assuming no bond character in the adjacent C—H bond is called No-Bond Resonance or Hyperconjugation.
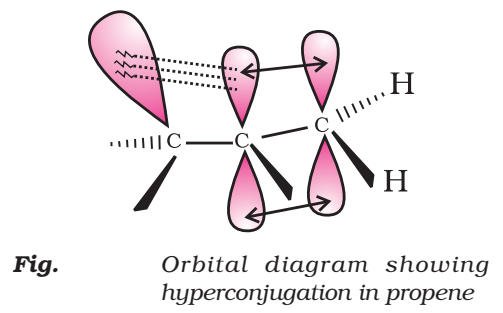
Orbital Concept of Hyperconjugation
It involves delocalization of o electrons of C—H bond of an alkyl group which is attached directly to an atom of unsaturated system or to an atom with an unshared p-orbital.
Let us consider CH3CH2 (ethyl cation) in which the positively charged carbon atom has an empty p-orbital. One of the C—H bonds of the methyl group can align in the plane of this empty p-orbital and electron constituting the C—H bond in plane with this p-orbital can then be delocalized into the empty p-orbital as in Fig.
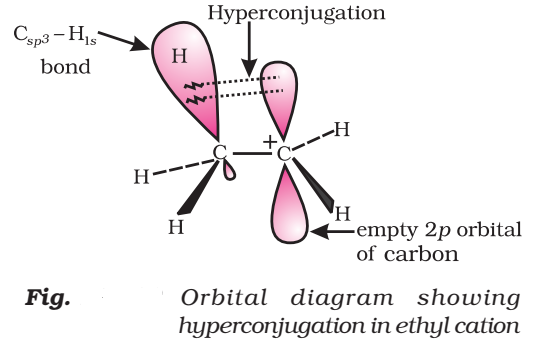
In general, greater the number of alkyl groups attached to a positively charged carbon atom, the greater is the hyperconjugation.