
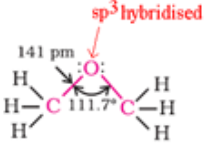
Ethers
Ethers are classified as simple or symmetrical, if the alkyl or aryl groups attached to the oxygen atom are the same, and mixed or unsymmetrical if the two groups are different. Diethyl ether, C2H5OC2H5, is a symmetrical ether whereas C2H5OCH3 and C2H5OC6H5 are unsymmetrical ethers.
Preparation of ethers:
Preparation of ethers By dehydration of alcohols (From alcohols)
Alcohols undergo dehydration in the presence of protic acids (H2SO4, H3PO4). The formation of the reaction product, alkene or ether depends on the reaction conditions.
![]()
Preparation of ethers Williamson synthesis (From alkyl halide and sodium alkoxide):
It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers. In this method, an alkyl halide is allowed to react with sodium alkoxide
Here, the alkyl halide should be primary and alkoxide should be tertiary. In case of aromatic ether, the aromatic part should be with phenoxide ion.
![]()

