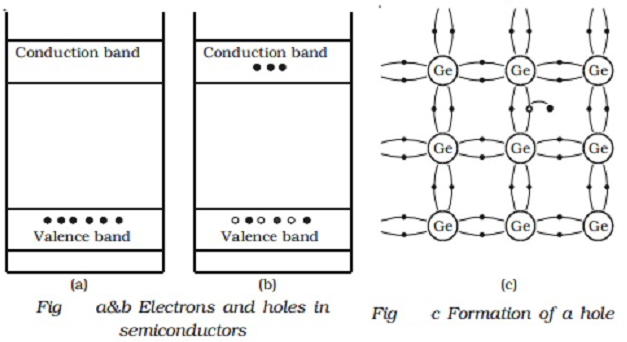
A semiconductor which is pure and contains no impurity is known as an intrinsic semiconductor. In an intrinsic semiconductor, the number of free electrons and holes are equal. Common examples of intrinsic semiconductors are pure germanium and silicon. Schematic band diagram of an intrinsic semiconductor at room temperature is represented in Fig.
Doping a semiconductor
Electrons and holes can be generated in a semiconductor crystal with heat energy or light energy. But in these cases, the conductivity remains very low. The efficient and convenient method of generating free electrons and holes is to add a very small amount of selected impurity inside the crystal. The impurity to be added is of the order of 100 ppm (parts per million). The process of addition of a very small amount of impurity into an intrinsic semiconductor is called doping. The impurity atoms are called dopants. The semiconductor containing impurity atoms is known as impure or doped or extrinsic semiconductor.
- The impurity atoms are added to the semiconductor in its molten state.
- The pure semiconductor is bombarded by ions of impurity atoms.
- When the semiconductor crystal containing the impurity atoms is heated, the impurity atoms diffuse into the host crystal.
- Usually, the doping material is either pentavalent atoms (bismuth, antimony, phosphorous, arsenic which has five valence electrons)
- Trivalent atoms (aluminium, gallium, indium, boron which have three valence electrons).
- The pentavalent doping atom is known as donor atom since it donates one electron to the conduction band of the pure semiconductor.
- The trivalent atom is called an acceptor atom because it accepts one electron from the pure semiconductor atom.

