Surface Chemistry is that branch of chemistry which deals with the study of the phenomena occurring at the surface or interface, i.e., at the boundary separating two bulk phases.
The two bulk phases can be pure compounds or solutions. The interface is represented by putting a hyphen between the two bulk phases involved, e.g., solid-liquid or solid/liquid. No interface exists between gases as they are completely miscible. Important phenomena occur at the interface, e.g., dissolution, crystallization, corrosion, heterogeneous catalysis, electrode processes, etc
Adsorption:
The phenomenon of attracting and retaining the molecules of a substance on the surface of a liquid or solid resulting in the higher concentration of the molecules on the surface is called adsorption. Adsorption of gases at metal surface is called occlusion.
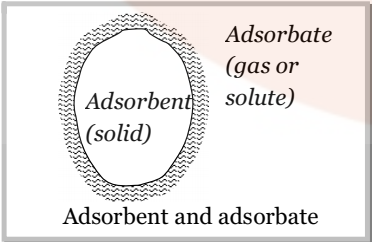
- Adsorbate: The molecular species or substance, which concentrates or accumulates at the surface is termed adsorbate.
Example: if a gas gets adsorbed on to the surface of a solid, then the gas is termed as the adsorbate.
- Adsorbent: The material on the surface of which the adsorption takes place is called adsorbent.
The adsorbent may be a solid or a liquid. Metal powders, powdered charcoal, animal charcoal silica powder etc. are commonly used as adsorbents.
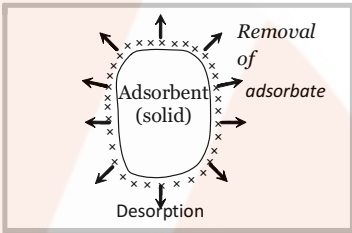
- Desorption: The removal of the adsorbed substance from a surface is called desorption. This can be done by heating or reducing the pressure of the system
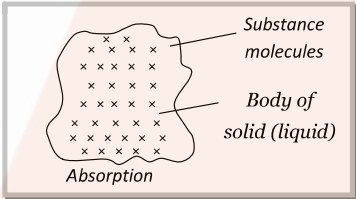
- Absorption: When the molecules of a substance are uniformly distributed throughout the body of a solid or liquid, this phenomenon is called absorption.
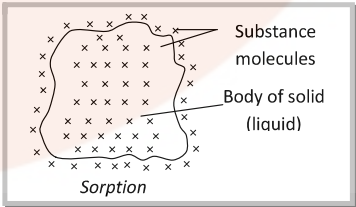
- Sorption: The phenomenon in which adsorption and absorption occur simultaneously is called sorption. Dyes are absorbed as well absorbed in cotton fibre.
Adsorption is instantaneous i.e. a fast process while absorption is a slow process.