
Isomers are two or more compounds that have the same chemical formula but a different arrangement of atoms.
Types of Isomerism
There are two types of isomerism:
- Stereoisomerism
- Geometrical isomerism
- Optical isomerism
- Structural isomerism
- Linkage isomerism
- Coordination isomerism
- Ionisation isomerism
- Hydration isomerism
Stereoisomerism
It arises due to the different arrangement of atoms or groups in space. Two different types of stereoisomerism are described as follows:
Geometrical isomerism:
It arises in heteroleptic complexes due to different possible geometrical arrangements of ligands. In square planar complex of formula [MX2L2] (where X and L are unidentate), the two ligands X may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans isomer.
For example: Geometrical isomers of Pt(NH3)2Cl2) are shown below:
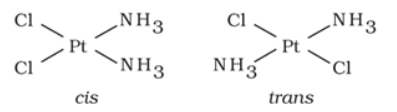
Similarly, in octahedral complexs of formula [MX2L4], two ligands X may be oriented cis or trans to each other.
For example: Geometrical isomers of [Co(NH3)4Cl2] are shown below:
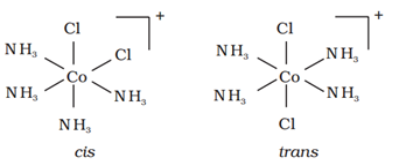
Optical isomerism:
It arises due to the presence of non-super imposable mirror images. The non-superimposable mirror images are called enantiomers. The enantiomers react differently with the plane polarised light.
The enantiomer which rotate the plane polarised light in a clockwise direction is called dextrorotatry (d) or (+) and the enantiomer which rotate the plane polarised light in anticlockwise direction is called levorotatory (l) or (‒).
For example: Optical isomers of [Co(en)3]3+ are shown below:
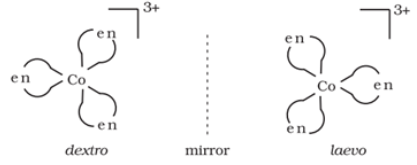
Structural isomerism
It arises due to the difference in structures of coordination compounds. Four different types of structural isomerism are described as follows:
Ionisation isomerism:
These are the isomers having the same molecular formula but gives different ions in solution.
For example: [Cr(NH3)Br]SO4 and [Cr(NH3)(SO4)]Br are ionization isomers.
Linkage isomerism:
Isomers having the same molecular formula but different linking atoms are named as linkage isomers. This arises due to the presence of ambident ligands.
For example: [CO(NH3)5(NO2)]2+ and [CO(NH3)5 (ONO)]2+ are linkage isomers.
Coordination isomerism:
This type of isomerism is possible when cation and anion both are complex and isomerism arises due to complete exchange of coordination sphere.
For example: [Pt (NH3)4] [Ni (CN)4] and [Ni (NH3)4] [Pt (CN)4] are coordination isomers.
Hydration isomerism:
Isomers having the same molecular formula but different water molecules of hydration are known as hydration isomers of each other.
For example: [Cr (H2O)5Cl]Cl2.H2O and [Cr(H2O)4Cl2]Cl.2H2O are hydration isomers.

