
Two commercially important alcohols are methanol and ethanol.
Methanol, CH3OH
Preparation of Methanol:
Methanol is mainly produced by catalytic hydrogenation of carbon monoxide at high pressure and temperature and in the presence of ZnO – Cr2O3 catalyst.
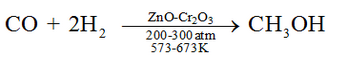
Properties of Methanol:
- It is a colourless liquid and highly poisonous.
- It is completely soluble in water.
Uses of Methanol:
- It is used as a solvent for paints, varnishes, and for making formaldehyde.
Ethanol, C2H5OH
Preparation of Ethanol:
Ethanol is mainly obtained commercially by fermentation of sugars. The sugar sugarcane or fruits such as grapes is converted to glucose and fructose, in the presence of an enzyme, invertase. Glucose and fructose on fermentation in the presence of another enzyme, zymase, yield ethanol.

Properties of Ethanol:
- Ethanol is a colourless liquid.
- The boiling point of ethanol is higher than methanol.
Uses of Ethanol:
- It is used as s solvent in the paint industry.
- It is also used in the preparation of a number of carbon compounds.
Denaturation of alcohol
The commercial alcohol is made unfit for drinking by mixing in it some copper sulphate and pyridine. This is known as denaturation of alcohol.

