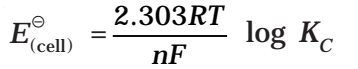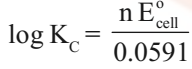Nernst Equation
It relates electrode potential with the concentration of ions. Thus, the reduction potential increases with the increase in the concentration of ions. For a general electrochemical reaction of the type.
![]()
Nernst equation can be given as
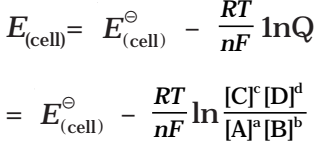
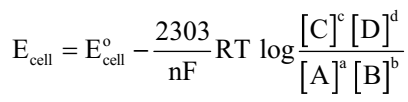
Substituting the values of R and F we get
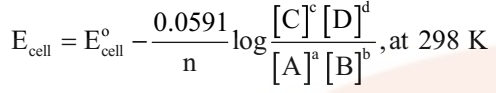
Application of Nernst Equation
Equilibrium Constant from Nernst Equation:
For a Daniel cell, at equilibrium
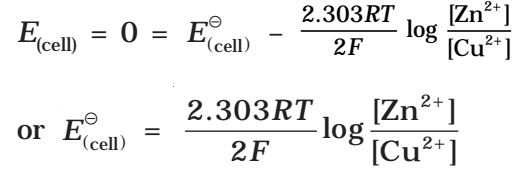
But at equilibrium,
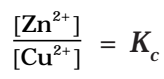
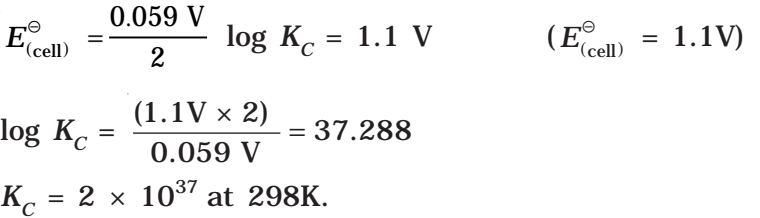
In general,