Osmosis Pressure (n)
Osmosis is the phenomenon of spontaneous flow of the solvent molecules through a semipermeable membrane from pure solvent to the solution or from a dilute solution to concentrated solution. It was first observed by Abbe Nollet.
Some natural semipermeable membranes are animal bladder, cell membrane etc.
CU2[Fe (CN)6] is an artificial semipermeable membrane which does not work in non-aqueous solutions as it dissolves in them.
Osmosis may be
- Exosmosis: It outward flows of water or solvent from a cell through a semipermeable membrane.
- Endosmosis: It is an inward flow of water or solvent from a cell through a semipermeable membrane.
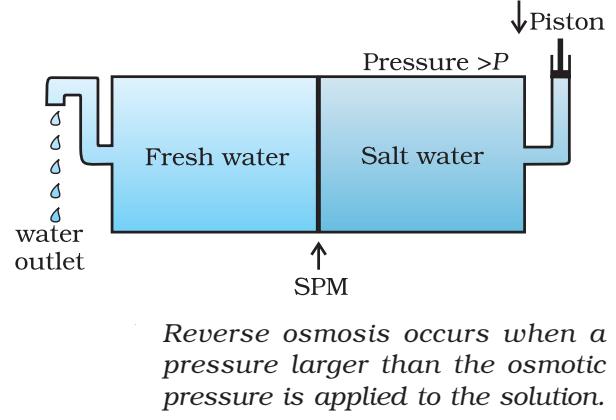
The hydrostatic pressure developed on the solution which just prevents the osmosis of pure solvent into the solution through a semipermeable membrane is called osmotic pressure.
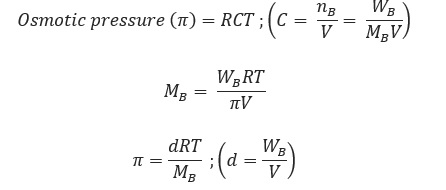
Where, d = density, R= solution constant,
T = temperature, MB = molar mass of solute
Osmotic pressure can be determined by anyone of the method listed below
- Pfeffer’s method
- Berkeley and Hartley’s method (very good method)
- Morse and Frazer’s method
On the basis of osmotic pressure, the solution can be
- Hypertonic solution: If we place the cells in a solution containing more than 0.9% (mass/volume) sodium chloride, water will flow out of the cells and they would shrink. Such a solution is called hypertonic.
- Hypotonic solution: If the salt concentration is less than 0.9% (mass/volume), the solution is said to be hypotonic. In this case, water will flow into the cells if placed in this solution and they would swell.
- Isotonic solution: Two solutions having same osmotic pressure at a given temperature are called isotonic solutions. When such solutions are separated by semipermeable membrane no osmosis occurs between them.
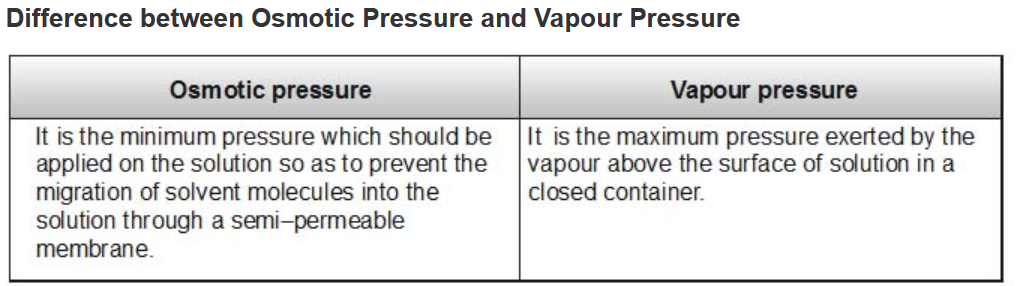
Reverse osmosis
The direction of osmosis can be reversed if a pressure larger than the osmotic pressure is applied to the solution side. That is, now the pure solvent flows out of the solution through the semipermeable membrane. This phenomenon is called reverse osmosis and is of great practical utility. Reverse osmosis is used in desalination of sea water. A schematic set up for the process is shown in Fig.