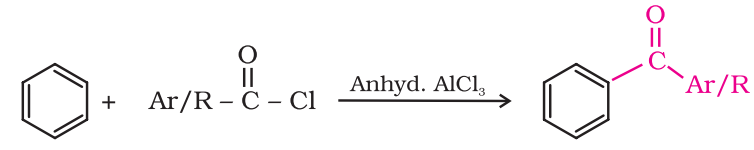Preparation of Aldehydes and Ketons
By oxidation of alcohols:
Aldehydes and ketones are generally prepared by oxidation of primary and secondary alcohols, respectively.
By dehydrogenation of alcohols:
This method is suitable for volatile alcohols and is of industrial application. In this method, alcohol vapours are passed over heavy metal catalysts (Ag or Cu). Primary and secondary alcohols give aldehydes and ketones, respectively.
From hydrocarbons
- By ozonolysis of alkenes: As we know, ozonolysis of alkenes followed by reaction with zinc dust and water gives aldehydes, ketones or a mixture of both depending on the substitution pattern of the alkene.
- By hydration of alkynes: Addition of water to ethyne in the presence of H2SO4 and HgSO4 gives acetaldehyde. All other alkynes give ketones in this reaction
Preparation of Aldehydes
Preparation of Aldehydes From acyl chloride (acid chloride)
Hydrogenation or acyl chloride over palladium on barium sulphate gives aldehyde. This reaction is called Rosenmund reduction.
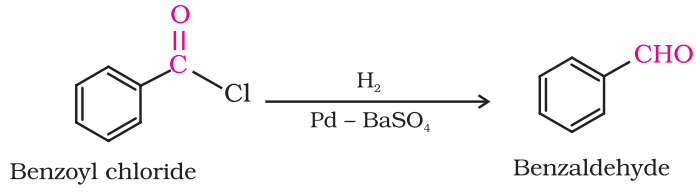
Preparation of Aldehydes From nitriles and esters
Nitriles are reduced to corresponding imine with stannous chloride in the presence of hydrochloric acid, which on hydrolysis give corresponding aldehyde.

This reaction is called Stephen reaction.
Esters are also reduced to aldehydes with DIBAL-H.
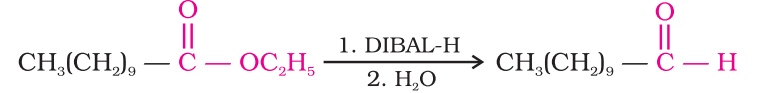
Preparation of Aldehydes From hydrocarbons
Preparation of Aldehydes By oxidation of methylbenzene:
Strong oxidising agents oxidise toluene and its derivatives to benzoic acids. The following methods are used for this purpose.
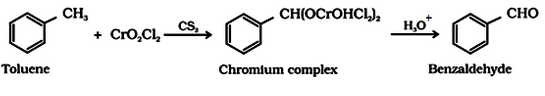
- Use of chromyl chloride (CrO2Cl2): This reaction is called Etard reaction.
- Use of chromic acid (CrO3): Toluene or substituted toluene is converted to benzylidene diacetate on treating with chromic oxide in acetic anhydride.
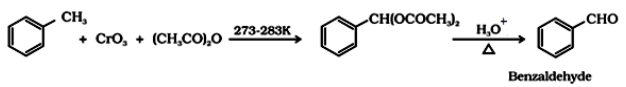
By side chain chlorination followed by hydrolysis:

This is a commercial method of manufacture of benzaldehyde.
By Gatterman – Koch reaction
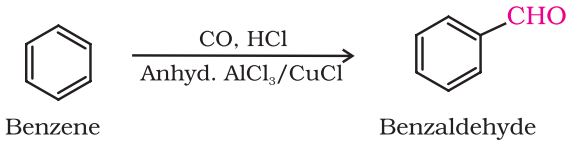
This reaction is known as Gatterman-Koch reaction.
Preparation of Ketones
Preparation of Ketones From acyl chlorides:
Treatment of acyl chlorides with dialkylcadmium, prepared by the reaction of cadmium chloride with Grignard reagent, gives ketones.

Preparation of Ketones From nitriles:
Treating a nitrile with Grignard reagent followed by hydrolysis yields
a ketone.
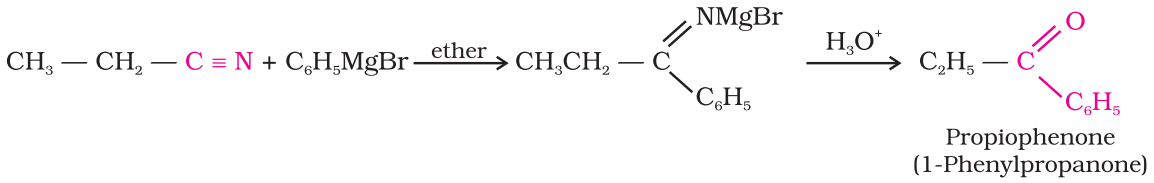
Preparation of Ketones From benzene or substituted benzenes:
When benzene or substituted benzene is treated with acid chloride in the presence of anhydrous aluminium chloride, it affords the corresponding ketone. This reaction is known as Friedel-Crafts acylation reaction.