
Primitive unit cell:
In a Primitive unit cell, atoms are present at the corner only. An atom is shared by 8 unit cell. Hence the only 1/8th of the atom belonging to the particular unit cell. There are total 8 corners in each unit cell, therefore,1/8 part of 8 atoms would give the value of the number of atoms per unit cell.
In brief 8 corners, 1 atom at each corner, 1/8 of each atom in unit cell
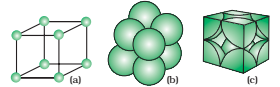
Centred unit cell (body- centred):
Body- centred unit cell:
The number of atoms present at the different position is as follows:
- 8 corners, 1 atom at each corner (1/8 of each atom in the unit cell)
- 1 atom at the centre of the body (1 atom completely present in the unit cell)

The total number of atom present in each unit cell:
= Contribution of atoms at corner + contribution of atom at the centre of the body
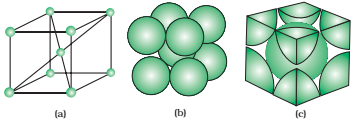
Face-centred unit cell:
The number of atoms present at the different position is as follows:
- 8 corners, 1 atom at each corner (1/8 of each atom in unit cell)
- 6 faces, 1 atom at each face of the unit cell (1/6; of each atom in the unit cell)


The total number of atom present in each unit cell:
= Contribution of atoms at corner + contribution of atom at the face of the body

