
Oxidation Processes: It involves in Corrosion, Bleaching, Antiseptics, Combustion of fuel.
Reduction Processes: It involves in Photography, Antioxidants, Photosynthesis, Metallurgy.
Electrochemical cells
Electrochemical cells are the devices which are used to get electric current by using chemical reaction.
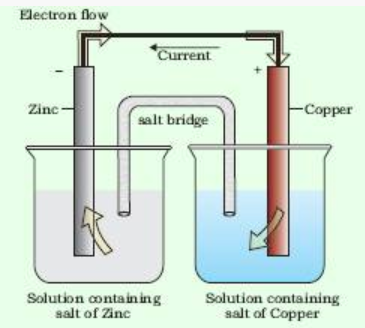
The potential associated with each electrode is known as electrode potential.
If the concentration of each species taking part in the electrode reaction is unity (if any gas appears in the electrode reaction, it is confined to 1 atmospheric pressure) and further their action is carried out at 298K, then the potential of each electrode is said to be the Standard Electrode Potential.
Its is used to measure electrode potential and its standard electrode potential is taken as 0.00V.

