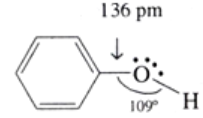
Structure of phenol
In phenols, the –OH group is attached to sp2 hybridised carbon of an aromatic ring. The C–O bond length (136 pm) in phenol is slightly less than that in methanol. This is due to (i) partial double bond character caused by the conjugation of unshared electron pair of oxygen with the aromatic ring and (ii) sp2 hybridised state of carbon to which oxygen is attached. The C−O−H bond angle in alcohols is slightly less than the tetrahedral angle (109°-28′) due to the repulsion between two lone pairs of electrons present on oxygen.