In solids, the atoms are arranged in a systematic space lattice and each atom is influenced by neighbouring atoms. The closeness of atoms results in the intermixing of electrons of neighbouring atoms. Due to this, the number of permissible energy levels increases.
Hence in the case of a solid, instead of a single energy level associated with a single atom, there will be bands of energy levels. A set of such closely packed energy levels is called an energy band.
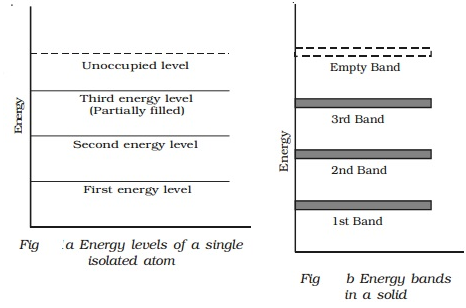
The concept of energy bands can be understood from Fig a and Fig b. The energy levels of a single isolated atom of silicon are shown in Fig a.
Each silicon atom has 14 electrons, two of which occupy K shell, 8 occupy the L shell and 4 occupy the M shell. The electrons in the M shell are distributed as 2 electrons in the subshell 3s and 2 electrons in the subshell 3p. This subshell 3p is partially filled because it can accommodate a total of 6 electrons. The completely filled levels are known as core levels and the electrons filling these levels are called core electrons. The electrons in the outermost level are called valence electrons. The partially filled outermost level is valence level and the permitted levels which are vacant are known as conduction levels.
In a solid, there are large numbers of atoms, which are very close to each other. The energy of s or p level is of the order of eV, therefore the levels are very closely spaced. The first orbit electrons form a band called first energy band. Similarly, second orbit electrons from second energy band and so on as shown in Fig b.

