
Potassium dichromate, K2Cr2O7
Preparation of Potassium dichromate:
It is prepared by fusion of chromate ore (FeCr2O4) with sodium carbonate in excess of air.
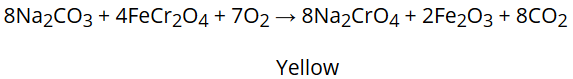
Na2CrO4 produced in the above reaction is then acidified to get sodium dichromate, Na2Cr2O7
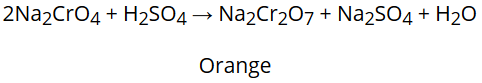
The solution of sodium dichromate treated with potassium chloride to get the final product, K2Cr2O7.

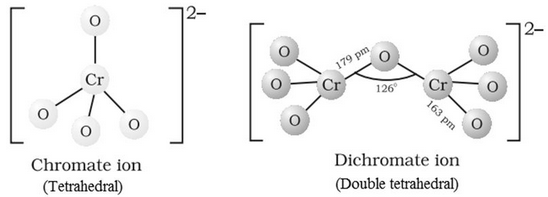
Structures of CrO42‒ and Cr2O72‒ ions:
Uses of Potassium dichromate:
Potassium dichromate is used as a primary standard in volumetric analysis and as an oxidizing agent. In acidic medium, the oxidation state of Cr changes from + 6 in Cr2O72‒ to + 3 in Cr3+.
![]()

