
Chemical Bond
The attractive force which holds various constituents (atoms, ions, etc.) together in different chemical species is called a chemical bond.
Modes of Chemical Composition
This can occur in two ways:
- By complete transference of one or more electrons from one atom to another. This process is referred to as electrovalency and the chemical bond formed is termed as an electrovalent bond or ionic bond.
- By sharing of electrons. This can occur in two ways as follows:
- When the shared electrons are contributed by the two combining atoms equally, the bond formed is called the covalent bond.
- When these electrons are contributed entirely by one of the atoms but shared by both, the bond formed is known as a coordinate bond, also called dative bond.
Kossel-Lewis Approach to Chemical Bonding
According to this theory, atom takes part in the bond formation to complete their octet or to acquire the electronic configuration of the nearest inert gas atoms (Octet rule). This can be achieved by gaining, losing or sharing the electrons.
Lewis Symbols
Valence electrons are reported by dots around the chemical symbol of element, e.g.,
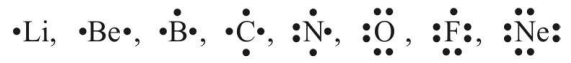
Octet Rule
During a chemical reaction, the atoms tend to adjust their electronic arrangement in such a way that they achieve 8 e– in their outermost electron. This is called the octet rule.
Formal Charge
The formal charge on an atom in a molecule or ion is defined as the difference between the number of valence electrons of that atom in the free state and the number of electrons assigned to that atom in the Lewis structure, assuming that in each shared pair of electrons, the atom has one electron of its own and the lone pair on it belongs to it completely. Thus, it can be calculated as follows:


