
Enthalpy (H)
It is defined as total heat content of the system. it is equal to the sum of internal energy and pressure-volume work.
Mathematically,
H = U + PV
Change in enthalpy:
Change in enthalpy is the heat absorbed or evolved by the system at constant pressure.
∆H = qp
For exothermic reaction (System loses energy to Surroundings),
∆H and qp both are -ve.
For endothermic reaction (System absorbed energy from the surrounding).
∆H and qp both are +ve.
Factors affecting enthalpy of reaction
- Physical state of reactants and products.
- Allotropic forms of elements involved.
- Chemical composition of reactants and products.
- Amount of reactants.
- Temperature.
Relation between ∆H and ∆U
Let us consider a general reaction A → B
Let HA be the enthalpy of reactant A and HB be that of the products.
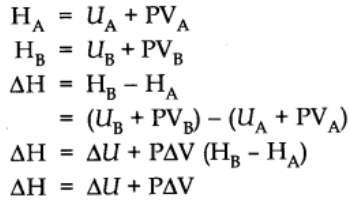
At constant pressure and temperature using ideal gas law,
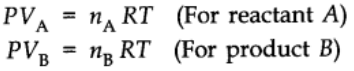
Thus,
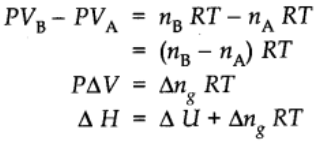
Extensive property
An extensive property is a properly whose value depends on the quantity or size of matter present in the system.
For example: Mass, volume, enthalpy etc are known as extensive property.
Intensive property
Intensive properties do not depend upon the size of the matter or quantity of the matter present in the system.
For example: temperature, density, pressure etc. are called intensive properties.

