Fluorine is anomalous in many properties like, ionisation enthalpy, electronegativity, enthalpy of bond dissociation that are higher than expected from the regular trends among the halogens. Its ionic and covalent radii, melting and boiling points, and electron gain enthalpy is quite lower than expected.
Reasons for the anomalous behaviour of Fluorine are:
- Small size and highest electronegativity
- Low F-F bond dissociation enthalpy
- Absence of d-orbitals
Reactivity towards hydrogen:
All halogens react with H2 to form hydrogen halides (HX) and the reactivity towards H2 decreases down the group.
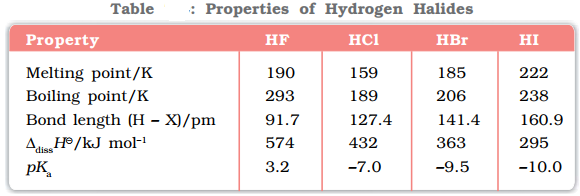
- Acidic strength: As the size of X increases and the strength of H─X bond decreases down the group, acidic strength decreases down the group.
![]()
- Stability: As the bond dissociation enthalpy decreases down the group so the stability of hydrogen halides also decreases from HF to HI.
![]()
- Boiling Point: Due to the increase in the size of halogens the van der Waals forces increases down the group resulting in the increase in boiling point from HCl to HI. HF has the highest boiling point due to the presence of strong intermolecular H bonding
![]()
- Ionic character: Due to the decrease in electronegativity down the group the ionic character of hydrogen halides also decreases down the group.
![]()
- Dipole Moment: Due to the decrease in electronegativity down the group the ionic character of hydrogen halides also decreases down the group.
![]()
- Reducing Power: As the bond dissociation enthalpy decreases, so it becomes easier to give out the hydrogen atom causing the reducing power to increase from HF to HI.
![]()
Reactivity towards oxygen:
- Halogens form many oxides with oxygen but most of them are unstable.
- Chlorine, bromine and iodine form oxides in which the oxidation states of these halogens range from +1 to +7.
- A combination of kinetic and thermodynamic factors leads to the generally decreasing order of stability of oxides formed by halogens, I > Cl > Br.
- The higher oxides of halogens tend to be more stable than the lower ones.
| Oxidation state | Fluorine | Chlorine | Bromine | Iodine |
|---|---|---|---|---|
| -1 | OF2 | |||
| +1 | Cl2O | Br2O | ||
| +2 | O2F2 | |||
| +4 | ClO2 | BrO2 | I2O4 | |
| +5 | I2O6 | |||
| +6 | Cl2O6 | BrO3 | ||
| +7 | Cl2O7 | I2O7 |
Reactivity towards metals:
Halogens react with metals to form metal halides of the form MX, where M is a monovalent metal.
- Ionic character: Due to the decrease in electronegativity down the group the ionic character of metal halides also decreases down the group
![]()
Reactivity of halogens towards other halogens (Interhalogens):
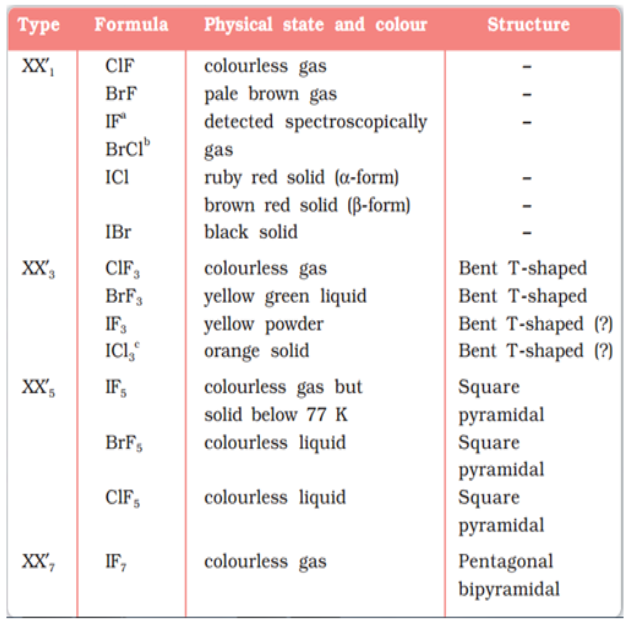
Binary compounds of two different halogen atoms of general formula XX’n are called interhalogen compounds where n = 1, 3, 5, or 7. All the interhalogen compounds are covalent in nature.
Some properties of interhalogen compounds are given in the following table: