
Buffer Solutions
The solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali, are called Buffer solutions. e.g.,
![]()
Acidic Buffer:
Weak acid and its salt with strong base are known as acidic buffer. pH < 7.
e.g., CH3COOH, CH3COONa, etc.
Basic Buffer:
Weak base and its salt with strong acid are known as basic buffer. pH > 7.
e.g., NH4Cl and NH4OH
Solubility Products
It is applicable to sparingly soluble salt. There is equilibrium between ions and unionized solid substance.
Consider an equation
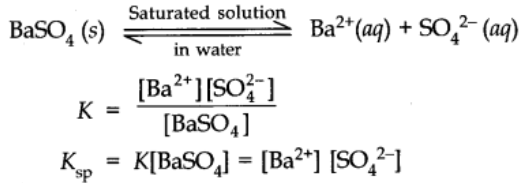
Ksp is called the solubility product constant or simply solubility product.
Under equilibrium conditions
![]()
The solubility of salts of weak acids like, phosphates, increases at lower pH.

