
Catalysis
Substances, which alter the rate of a chemical reaction and themselves remain chemically and quantitatively unchanged after the reaction, are known as catalysts, and the phenomenon is known as catalysis.
Catalytic reactions can be broadly divided into the following types:
- Homogeneous catalysis
- Heterogeneous catalysis
Homogeneous catalysis:
When the reactants and the catalyst are in the same phase (i.e. solid, liquid or gas). The catalysis is said to be homogeneous. The following are some of the examples of homogeneous catalysis.
- Oxidation of sulphur dioxide into sulphur trioxide with dioxygen in the presence of oxides of nitrogen as the catalyst in the lead chamber process.
![]()
The reactants, sulphur dioxide and oxygen, and the catalyst, nitric oxide, are all in the same phase.
- Hydrolysis of methyl acetate is catalysed by H+ ions furnished by hydrochloric acid.
![]()
Both the reactants and the catalyst are in the same phase.
- Hydrolysis of sugar is catalysed by H+ ions furnished by sulphuric acid.

Both the reactants and the catalyst are in the same phase
Heterogeneous catalysis:
The catalytic process in which the reactants and the catalyst are in different phases is known as heterogeneous catalysis. Some of the examples of heterogeneous catalysis are given below.
- Oxidation of sulphur dioxide into sulphur trioxide in the presence of Pt.
![]()
The reactant is in gaseous state while the catalyst is in the solid state.
- Combination between dinitrogen and dihydrogen to form ammonia in the presence of finely divided iron in Haber’s process.
![]()
The reactants are in gaseous state while the catalyst is in the solid state.
- Oxidation of ammonia into nitric oxide in the presence of platinum gauze in Ostwald’s process.
![]()
The reactants are in gaseous state while the catalyst is in the solid state.
- Hydrogenation of vegetable oils in the presence of finely divided nickel as catalyst.
![]()
One of the reactants is in liquid state and the other in gaseous state while the catalyst is in the solid state.
Important features of solid catalysts:
- Activity: Activity is the ability of catalysts to accelerate chemical reactions. The degree of acceleration can be as high as 10 10 times in certain reactions. For example, reaction between H2 and O2 to form H2O in presence of platinum as catalyst takes place with explosive violence. In absence of catalyst, H2 and O2 can be stored indefinitely without any reaction.
![]()
- Selectivity: Is the ability of catalysts to direct reaction to yield particular products (excluding other).
For example, starting with H2 and CO, and using different catalysts, we get different products.

Enzyme Catalysis
Enzymes are complex nitrogenous substances secreted by low forms of vegetables & organisms. Enzymes are actually protein molecules of higher molecular mass. (ranging from 15,000 – 1,000,000 g/mol) Enzymes form colloidal solutions in water and are very effective catalysts. They catalyse numerous reactions, especially those connected with natural processes. Numerous reactions occur in the bodies of animals and plants to maintain the life process. These reactions are catalysed by enzymes. The enzymes are thus, termed as biochemical catalysts and the phenomenon is known as biochemical catalysis.
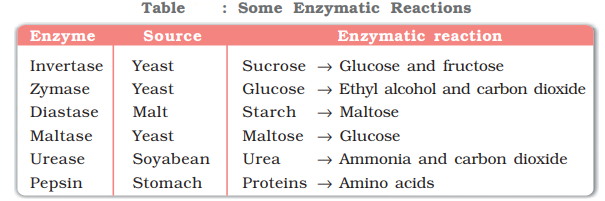
- Inversion of cane sugar: The invertase enzyme converts cane sugar into glucose and fructose.

- Conversion of glucose into ethyl alcohol: The zymase enzyme converts glucose into ethyl alcohol and carbon dioxide.
![]()
- Conversion of starch into maltose: The diastase enzyme converts starch into maltose.

- Conversion of maltose into glucose: The maltase enzyme converts maltose into glucose.

- Decomposition of urea into ammonia and carbon dioxide: The enzyme urease catalyses this decomposition.
![]()
- In stomach, the pepsin enzyme converts proteins into peptides while in intestine, the pancreatic trypsin converts proteins into amino acids by hydrolysis.
- Conversion of milk into curd: It is an enzymatic reaction brought about by lacto bacilli enzyme present in curd.
Characteristics of enzyme catalysis:
Enzyme catalysis is unique in its efficiency and high degree of specificity. The following characteristics are exhibited by enzyme catalysts:
- Most highly efficient
- Highly specific nature
- Highly active under optimum temperature
- Highly active under optimum pH
- Increasing activity in presence of activators and co-enzymes
- Influence of inhibitors and poisons
Mechanism of enzyme catalysis:
There are a number of cavities present on the surface of colloidal particles of enzymes. These cavities are of the characteristic shape and possess active groups such as -NH2, -COOH, -SH, -OH, etc. These are actually the active centers on the surface of enzyme particles. The molecules of the reactant (substrate), which have complementary shape, fit into these cavities just like a key fit into a lock. On account of the presence of active groups, an activated complex is formed which then decomposes to yield the products.

Thus, the enzyme-catalysed reactions may be considered to proceed in two steps.
Step1: Binding of enzyme to substrate to form an activated complex.
![]()
Step2: Decomposition of the activated complex to form product.
![]()


