
Chemical Properties and Trends in Chemical Reactivity
- Like the first member of other groups, Nitrogen differs from the rest of the members of this group due to its smaller size, high electronegativity, high ionization enthalpy and non-availability of d orbitals.
- Nitrogen has unique ability to form pπ–pπ multiple bonds with itself and with other elements having the small size and high electronegativity (e.g., C, O).
- Heavier elements of this group do not form pπ-pπ bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping.
- Thus, nitrogen exists as a diatomic molecule with a triple bond (one s and two p) between the two atoms.
- On the contrary, phosphorus, arsenic and antimony form metallic bonds in the elemental state.
- However, the single N – N bond is weaker than the single P – P bond because of high interelectronic repulsion of the non – bonding electrons, owing to the small bond length.
- As a result, the catenation tendency is weaker in nitrogen. Another factor which affects the chemistry of nitrogen is the absence of d orbitals in its valence shell.
Reactivity towards hydrogen:
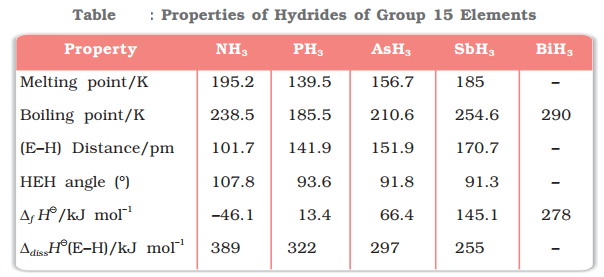
All the elements of Group 15 form hydrides of the type EH3 where E = N, P, As, Sb or Bi. Such as:
![]()
Nitrogen also forms a special hydride of the formula HN3, known as hydrogen azide or hydrazoic acid.
Reactivity towards oxygen:
All these elements form two types of oxides: E2O3 and E2O5.
The oxide in the higher oxidation state of the element is more acidic than that of lower oxidation state. Their acidic character decreases down the group.
The oxides of the type E2O3 of nitrogen and phosphorus are purely acidic, that of arsenic and antimony amphoteric and those of bismuth is predominantly basic.

Reactivity towards halogens:
These elements react to form two series of halides: EX3 and EX5. Nitrogen does not form pentahalide due to non-availability of the d orbitals in its valence shell. Pentahalides are more covalent than trihalides. All the trihalides of these elements except those of nitrogen are stable. In case of nitrogen, only NF3 is known to be stable. Trihalides except BiF3 are predominantly covalent in nature.
Reactivity towards metals:
All these elements react with metals to form their binary compounds exhibiting –3 oxidation state, such as Ca3N2 (calcium nitride) Ca3P2 (calcium phosphide), Na3As2 (sodium arsenide), Zn3Sb2 (zinc antimonide) and Mg3Bi2 (magnesium bismuthide).

