Chemical Reactions of Halides
- The reaction of haloalkanes: The reactions of haloalkanes may be divided into the following categories:
- Nucleophilic substitution: A nucleophile attacks the haloalkane which is having a partial positive charge on the carbon atom bonded to halogen. Halide ion separates following a substitution reaction.

Reactivity of haloalkanes towards nucleophilic substitution:
For the same alkyl group, as we move from F to I, strength of C−X bond decreases, therefore, the reactivity order is:
R− I > R−Br > R−Cl > R−F
Mechanism of nucleophilic substitution reaction:
This reaction has been found to proceed by two different mechanism which are described below:

Substitution nucleophilic bimolecular (SN2):
This type of nucleophilic substitution takes place in one step. The incoming nucleophile interacts with alkyl halide causing the C−X bond to break while forming a new C−OH bond.
The reactivity of alkyl halide towards SN2 reaction is:
![]()
Substitution nucleophilic unimolecular (SN1):
This type of nucleophilic substitution takes place in two steps, first step being the rate determining step involves the formation of carbonium ion.
The reactivity order of haloalkanes towards SN1reaction is:
![]()
Stereochemical aspects of nucleophilic substitution reactions:
A SN2 reaction proceeds with complete stereochemical inversion while a SN1 reaction proceeds with racemisation.
To understand this concept, we need to learn some basic stereochemical principles and notations (optical activity, chirality, retention, inversion, racemisation, etc.).
Plane polarized light and optical activity:
Certain compounds rotate the plane polarised light (produced by passing ordinary light through Nicol prism) when it is passed through their solutions. Such compounds are called optically active compounds. The angle by which the plane polarised light is rotated is measured by an instrument called polarimeter.
If the light is rotated towards left (anticlockwise direction) laevorotatory or the 1-form or negative – sign placed.
If the light is rotated towards right (clockwise direction) dextrorotatory or the d-form or positive + sign placed.
Molecular asymmetry, chirality and enantiomers:
The spatial arrangement of four groups (valencies) around a central carbon is tetrahedral and if all the substituents attached to that carbon are different, such a carbon is called asymmetric carbon or stereocentre.
The objects which are nonsuperimposable on their mirror image (like a pair of hands) are said to be chiral and this property is known as chirality. While the objects, which are, superimposable on their mirror images are called achiral.

The stereoisomers related to each other as non-superimposable mirror images are called enantiomers.
Enantiomers possess identical physical properties namely, melting point, boiling point, solubility, refractive index, etc. They only differ with respect to the rotation of plane polarised light. If one of the enantiomer is dextrorotatory, the other will be laevorotatory.
Retention:
Retention of configuration is the preservation of integrity of the spatial arrangement of bonds to an asymmetric centre during a chemical reaction or transformation. It is also the configurational correlation when a chemical species XCabc is converted into the chemical species YCabc having the same relative configuration.

Inversion, retention and racemistion:
There are three outcomes for a reaction at an asymmetric carbon atom. Consider the replacement of a group X by Y in the following reaction;
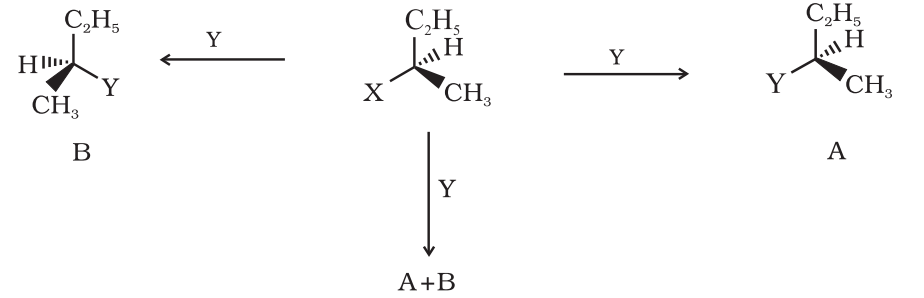
If (A) is the only compound obtained, the process is called retention of configuration.
If (B) is the only compound obtained, the process is called inversion of configuration.
If a 50:50 mixture of the above two is obtained then the process is called racemisation and the product is optically inactive, as one isomer will rotate light in the direction opposite to another.
Elimination reactions:
When a haloalkane with β-hydrogen atom is heated with alcoholic solution of potassium hydroxide, there is elimination of hydrogen atom from β-carbon and a halogen atom from the α-carbon atom resulting in the formation of an alkene. The reaction follows the Saytzeff rule which states that “In dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.”

Reaction with metals:
Reaction with Magnesium:
Alkyl halides react with magnesium in the presence of dry ether to form corresponding alkyl magnesium halide which is also known as Grignard’s reagent.

Reaction with sodium:
Alkyl halides react with sodium to form an alkane with double number of carbon atom than that present in alkyl halide. This reaction is also known as Wurtz reaction.
![]()
Reaction of Haloarenes
Nucleophilic substitution:
Aryl halides are extremely less reactive towards nucleophilic substitution reactions due to the following reasons:
Resonance effect:
Aryl halides are almost unreactive towards nucleophilic substitution reaction. This is because of double character of C – X bond due to resonance. Therefore, it is difficult to remove X from C – X bond.

Difference in hybridisation of carbon atom in C-X bond:
In haloalkane, the carbon atom attached to halogen is sp3 hybridised while in case of haloarene, the carbon atom attached to halogen is sp2-hybridised.

- Instability in phenyl cation: In case of haloarenes, the phenyl cation formed because of self-ionisation will not be stabilized by resonance and therefore, SN1 mechanism is ruled out.
- Because of the possible repulsion, it is less likely for the electron rich nucleophile to approach electron rich arenes.
Replacement by hydroxyl group:
Chlorobenzene can be converted into phenol by heating in aqueous sodium hydroxide solution at a temperature of 623K and a pressure of 300 atmospheres.
The presence of an electron withdrawing group (-NO2) at ortho- and para- positions increases the reactivity of haloarenes.

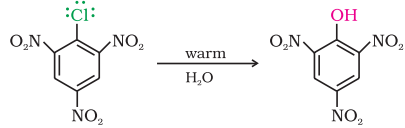
NO2 group increases the reactivity more when present at o- and p- position due to the increased delocalization of negative charge involving NO2 group.
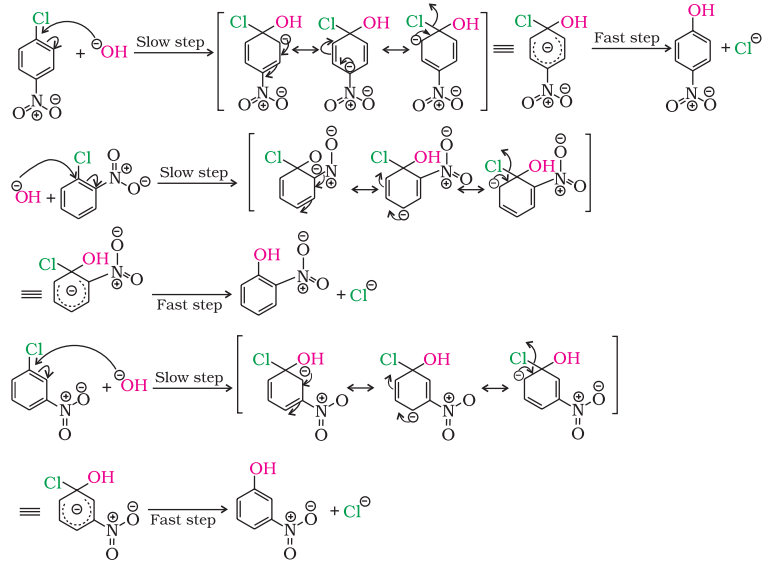
Electrophilic substitution reactions:
Haloarenes undergo the usual electrophilic reactions of the benzene ring such as halogenation, nitration, sulphonation and Friedel-Crafts reactions. Halogen atom besides being slightly deactivating is o, p directing; therefore, further substitution occurs at ortho- and para- positions with respect to the halogen atom. The o, p-directing influence of halogen atom can be easily understood if we consider the resonating structures of halobenzene as shown:

Halogenation
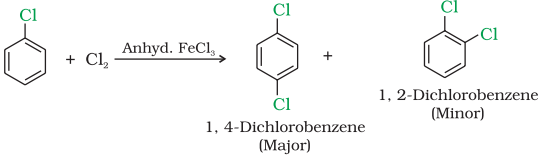
Nitration
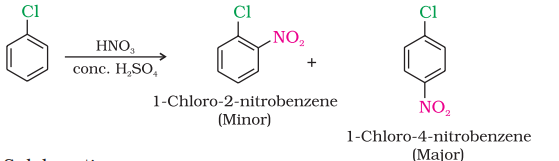
Sulphonation

Friedel-Crafts reaction
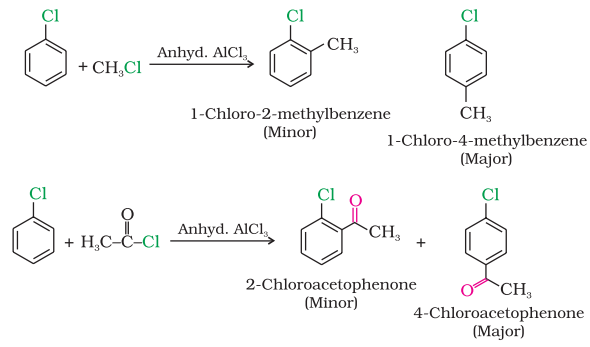
Reaction with metals:
Wurtz-Fittig reaction:
A mixture of an alkyl halide and aryl halide gives an alkylarene when treated with sodium in dry ether and is called Wurtz-Fittig reaction.

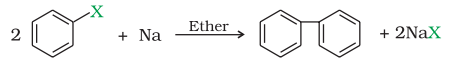
Fittig reaction:
Aryl halides also give analogous compounds when treated with sodium in dry ether, in which two aryl groups are joined together. It is called Fittig reaction.