
Classification of Polymers:
There are several ways of classification of polymers based on some special considerations.
Based on Source:
Under this type of classification, there are three subcategories.
- Natural polymers: These polymers are found in plants and animals. Examples are proteins, cellulose, starch, resins and rubber.
- Semi-synthetic polymers: Cellulose derivatives as cellulose acetate (rayon) and cellulose nitrate, etc. are the usual examples of this subcategory.
- Synthetic polymers: A variety of synthetic polymers as plastic (polythene), synthetic fibres (nylon 6,6) and synthetic rubbers (Buna – S) are examples of manmade polymers extensively used in daily life as well as in industry.
Based on Structure of Polymers:
There are three different types based on the structure of the polymers.
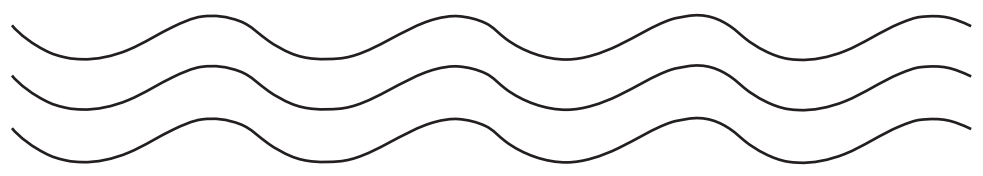
- Linear polymers: These polymers consist of long and straight chains. The examples are
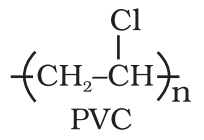
high-density polyethene, polyvinyl chloride, etc. These are
represented as:
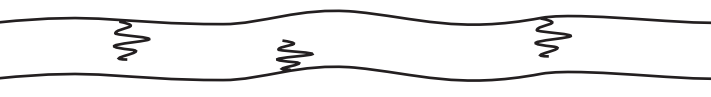
- Branched-chain polymers: These polymers contain linear chains having some branches, e.g., low-density polythene. These are depicted as follows:
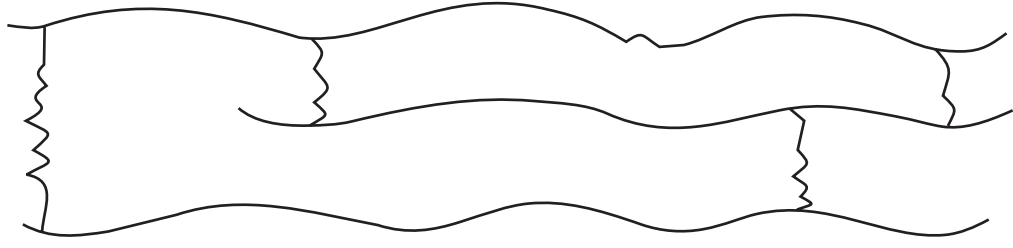
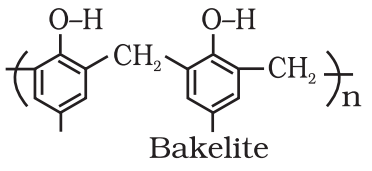
- Cross-linked or Network polymers: These are usually formed from bi-functional and tri-functional monomers and contain strong covalent bonds between various linear polymer chains, e.g. bakelite, melamine, etc. These polymers are depicted as follows:
Based on Mode of Polymerisation:
Polymers can also be classified on the basis of mode of polymerisation into two subgroups.
- Addition polymers: The addition polymers are formed by the repeated addition of monomer molecules possessing double or triple bonds, e.g., the formation of polythene from ethene and polypropene from propene. However, the addition polymers formed by the polymerisation of a single monomeric species are known as homopolymers, e.g., polythene.
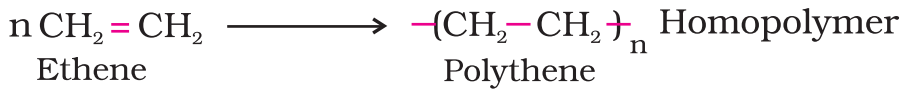
The polymers made by addition polymerisation from two different monomers are termed as copolymers, e.g., Buna-S, Buna-N, etc.
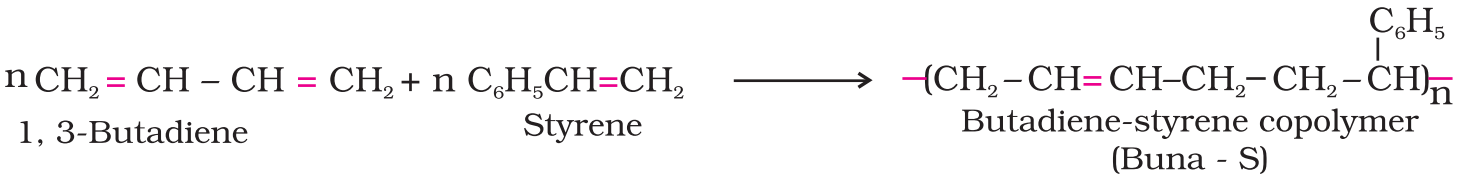
- Condensation polymers: Condensation polymers are formed by the combination of monomers with the elimination of simple molecules such as water or alcohol. This process is called condensation polymerisation. Proteins, starch, cellulose etc. are the example of natural condensation polymers.
Two main synthetic polymers of condensation types are polyesters (Terylene or dacron) and polyamides (Nylon-66).
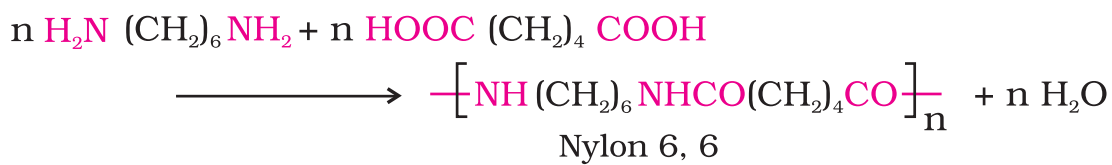
Based on Molecular Forces:
Under this category, the polymers are classified into the following four subgroups on the basis of the magnitude of intermolecular forces present in them.
- Condensation polymers: These are the polymers having very weak intermolecular forces of attraction between polymer chains. Elastomers possesses elastic character. Vulcanized rubber is very important example of an elastomer.
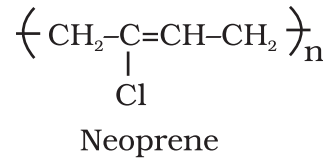
- Fibers: Fibers are the thread forming solids which possess high tensile strength and high modulus. These characteristics can be attributed to the strong intermolecular forces like hydrogen bonding. These strong forces also lead to close packing of chains and thus impart crystalline nature. The examples are polyamides (nylon 6, 6), polyesters (terylene), etc.
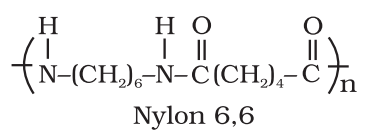
- Thermoplastics: A thermoplastic polymer is one which softens on heating and becomes hard on cooling. Polyethylene, polypropylene, polystyrene is the example of thermoplastics.
- ThermoSetting Polymers or Resin: A thermosetting polymer becomes hard on heating. Bakelite, Aniline aldehyde resin, urea formaldehyde polymer.
Based on Growth Polymerisation:
There are two different types based on Growth of Polymerisation.
- Chain-Growth Polymerization: These polymers are formed by the successive addition of monomer units to the growing chain having a reactive intermediate (Free radical, carbocation or carbanion). Chain growth polymerisation is an important reaction of alkenes and conjugated dienes.
Polythene, polypropylene, teflon, PVC, polystyrene are some examples of chain growth polymers. - Step-Growth Polymerization: These polymers are formed through a series of independent steps. Each step involves the condensation between two monomers leading to the formation of smaller polymer. e.g. Nylon, terylene, bakelite etc.
Based on Structure:
There are two different types based on structure.
- Linear Polymers: These consist of extremely long chains of atoms and are also called one-dimensional polymers.
Examples – Polyethylene, PVC, Nylon, Polyester. - Three-Dimensional Polymers: Those polymers in which chains are cross-linked to give a three-dimensional network are called three-dimensional polymers.
Example – Bakelite.

