
Colloids
The colloidal state depends on the particle size. It is regarded as an intermediate state between true solution and suspension.
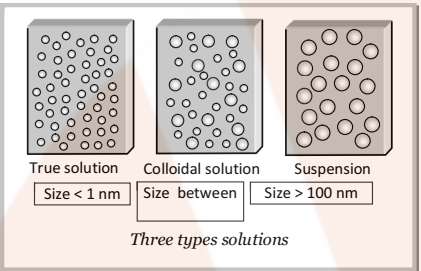
True Solution:
In true solution, the size of the particles of solute is very small and thus, these cannot be detected by any optical means and freely diffuse through membranes. It is a homogenous system.
Suspension:
The size of particles is large and, thus it can be seen by naked eye and do not pass through filter paper. It is a heterogeneous system.
Classification of colloids:
Colloids are classified on the basis of the following criteria:
- The physical state of the dispersed phase and the dispersion medium
- Nature of interaction between dispersed phase and dispersion medium.
- Type of particles of the dispersed phase.
Classification Based on the Physical state of Dispersed Phase and Dispersion Medium:
Depending upon whether the dispersed phase and the dispersion medium are solids, liquids or gases, eight types of colloidal systems are possible. A gas mixed with another gas forms a homogeneous mixture and hence is not a colloidal system. The examples of the various types of colloids along with their type names are listed in Table:

Classification based on Nature of interaction between dispersed phase and dispersion medium:
- Lyophilic colloids (water-loving): “The colloidal solutions in which the particles of the dispersed phase have a great affinity for the dispersion medium, are called lyophilic colloids.”
- Lyophobic colloids (water-hating): “The colloidal solution in which there is no affinity between particles of the dispersed phase and the dispersion medium are called lyophobic colloids.”

Classification based on types of particles of dispersed:
- Multimolecular colloids
- Macromolecular colloids
- Associated colloids

Micelles:
Micelles are the clusters or aggregated particles formed by the association of colloid in solution. The common examples of micelles are soaps and detergents. The formation of micelles takes place above a particular temperature called Kraft temperature(TK) and above a particular concentration called critical micellization concentration(CMC). They are capable of forming ions. Micelles may contain as many as 100 molecules or more
- For example, sodium stearate (C17H35COONa) is a typical example of such type of molecules.
- When sodium stearate is dissolved in water, it gives Na+ and C17H35COO– ions.

The stearate ions associate to form ionic micelles of colloidal size.
- It has long hydrocarbon part of C17H35 radical. Which is lyophobic and COO– part which is lyophilic.

Cleansing action of soaps
It has been mentioned earlier that a micelle consists of a hydrophobic hydrocarbon – like central core. The cleansing action of soap is due to the fact that soap molecules form the micelle around the oil droplet in such a way that hydrophobic part of the stearate ions is in the oil droplet and hydrophilic part projects out of the grease droplet like the bristles (Fig). Since the polar groups can interact with water, the oil droplet surrounded by stearate ions is now pulled in water and removed from the dirty surface. This soap helps in emulsification and washing away of oils and fats. The negatively charged sheath around the globules prevents them from coming together and forming aggregates.

Properties of Colloidal Solution:
- Colligative properties: Colloidal particles being bigger aggregates, the number of particles in a colloidal solution is comparatively small as compared to a true solution. Hence, the values of colligative properties (osmotic pressure, lowering in vapour pressure, depression in freezing point and elevation in boiling point) are of small order as compared to values shown by true solutions at same concentrations.
- Tyndall effect: When light passes through a sol, its path becomes visible because of scattering of light by particles. It is called Tyndall effect. This phenomenon was studied for the first time by Tyndall. The illuminated path of the beam is called Tyndall cone. In a true solution, there are no particles of sufficiently large diameter to scatter light and hence no Tyndall effect is observed. The Tyndall effect has also been observed by an instrument called ultramicroscope.
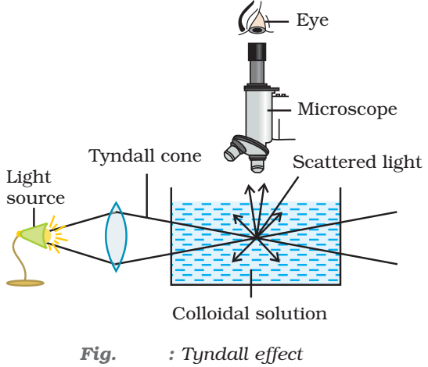
Tyndall effect is observed only when the following two conditions are satisfied
- The diameter of the dispersed particles is not much smaller than the wavelength of the light used; and
- The refractive indices of the dispersed phase and the dispersion medium differ greatly in magnitude.
- Colour: The colour of colloidal solution depends on the wavelength of light scattered by the dispersed particles. The wavelength of light further depends on the size and nature of the particles. The colour of colloidal solution also changes with the manner in which the observer receives the light.
- Brownian movement: When colloidal solutions are viewed under a powerful ultra-microscope, the colloidal particles appear to be in a state of continuous zig-zag motion all over the field of view. This motion was first observed by the British botanist, Robert Brown, and is known as the Brownian movement. This motion is independent of the nature of the colloid but depends on the size of the particles and viscosity of the solution. Smaller the size and lesser the viscosity, faster is the motion.

- The charge on colloidal particles: Colloidal particles always carry an electric charge. The nature of this charge is the same on all the particles in a given colloidal solution and may be either positive or negative.

Electrophoresis:
The phenomenon of movement of colloidal particles under an applied electric field is called electrophoresis. If the particles accumulate near the negative electrode, the charge on the particles is positive. On the other hand, if the sol particles accumulate near the positive electrode, the charge on the particles is negative.
When electrophoresis, i.e., movement of particles is prevented by some suitable means, it is observed that the dispersion medium begins to move in an electric field. This phenomenon is termed electro-osmosis.

Coagulation or precipitation:
“The phenomenon of the precipitation of a colloidal solution by the addition of the excess of an electrolyte is called coagulation or flocculation.”
The coagulation of the lyophobic sols can be carried out in the following ways:
- By electrophoresis: The colloidal particles move towards oppositely charged electrodes, get discharged and precipitated.
- By mixing two oppositely charged sols: Oppositely charged sols when mixed in almost equal proportions, neutralise their charges and get partially or completely precipitated. Mixing of hydrated ferric oxide (+ve sol) and arsenious sulphide (–ve sol) bring them in the precipitated forms. This type of coagulation is called mutual coagulation.
- By boiling: When a sol is boiled, the adsorbed layer is disturbed due to increased collisions with the molecules of dispersion medium. This reduces the charge on the particles and ultimately lead to settling down in the form of a precipitate.
- By persistent dialysis: On prolonged dialysis, traces of the electrolyte present in the sol are removed almost completely and the colloids become unstable and ultimately coagulate.
- By addition of electrolytes: When excess of an electrolyte is added, the colloidal particles are precipitated. The reason is that colloids interact with ions carrying charge opposite to that present on themselves. This causes neutralisation leading to their coagulation. The ion responsible for neutralization of charge on the particles is called the coagulating ion. A negative ion causes the precipitation of positively charged sol and vice versa.
- Coagulation of lyophilic sols: There are two factors which are responsible for the stability of lyophilic sols. These factors are the charge and solvation of the colloidal particles. When these two factors are removed, a lyophilic sol can be coagulated. This is done (i) by adding an electrolyte and (ii) by adding a suitable solvent.
- Protection of colloids: Lyophilic sols are more stable than lyophobic sols. This is due to the fact that lyophilic colloids are extensively solvated, i.e., colloidal particles are covered by a sheath of the liquid in which they are dispersed.
Lyophilic colloids have a unique property of protecting lyophobic colloids. When a lyophilic sol is added to the lyophobic sol, the lyophilic particles form a layer around lyophobic particles and thus protect the latter from electrolytes. Lyophilic colloids used for this purpose are called protective colloids.


Excellent explanation.
Please share the book’s name, from where the pictures were adopted.
Thanks for commenting. Images are adopted from old NCERT books.