
If the two bifunctional monomer molecules undergo condensation with loss of a simple molecule of water, alcohol etc.
Polyamides:
These polymers possessing amide linkages are important examples of synthetic fibres and are termed as nylons. The general method of preparation consists of the condensation polymerisation of diamines with dicarboxylic acids and also of amino acids and their lactams.
Preparation of nylons:
1. Nylon 6,6
It is manufactured by condensation polymerisation of adipic acid and hexamethylenediamine.
Reaction:
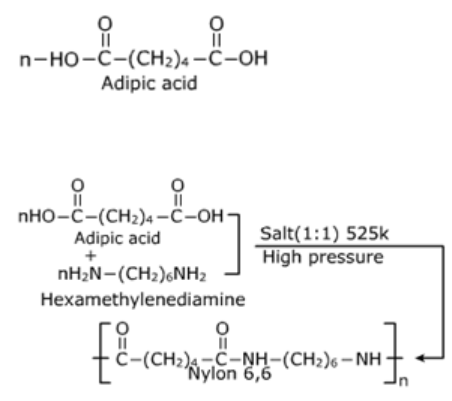
Nylon 6, 6 is used in making sheets, bristles for brushes and in textile industry.
2. Nylon 6:
It is obtained by heating caprolactam with water at a high temperature.

Nylon 6 is used for the manufacture of tyre cords, fabrics and ropes.
Polyesters:
Polymers which have the ester linkage are called polyesters and are prepared by condensation polymerisation of diacids and diols.
Reaction:
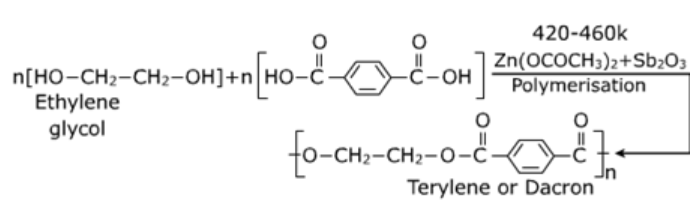
Properties:
- The fibre of terylene is highly crease-resistant.
- It is also not damaged by pests like moths or mildew.
Uses:
- It is used in the manufacture of wash and wears fabrics.
- It is used for making magnetic recording tapes.
Phenol-Formaldehyde Polymers (Bakelite and related polymers):
They are obtained by condensation of phenol with formaldehyde in presence of either an acid or abase catalyst the initial product is Navolac which is points.
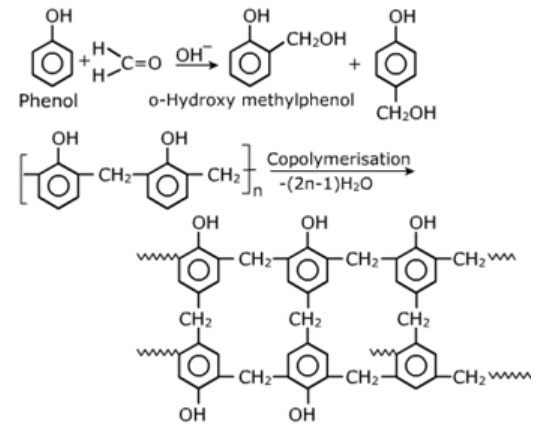
Melamine-Formaldehyde Polymers:
Melamine and formaldehyde undergo condensation copolymerization to form – melamine – Formaldehyde polymer also called me/mac.
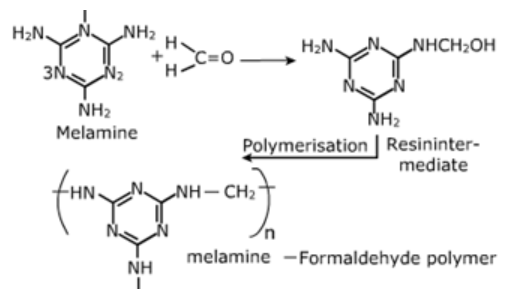
Uses:
- Melamine Copolymer is widely used for making non-breakable plastic crockery.
- Used for making clips and plates.
Copolymerisation
When two or more different monomers are allowed to polymeric together the product formed is called a polymer and the process is called copolymerization.
Example:
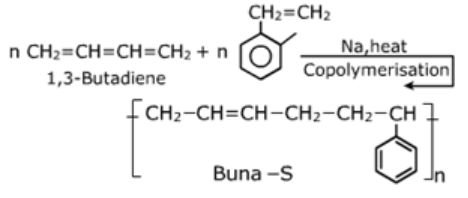
Properties of Copolymers:
- They have similar properties like alloying in mettalurgy.
- Polystyrene is a good electrical insulator.
- Buna – S have very fough, it possess very high abrasion resistance.

