
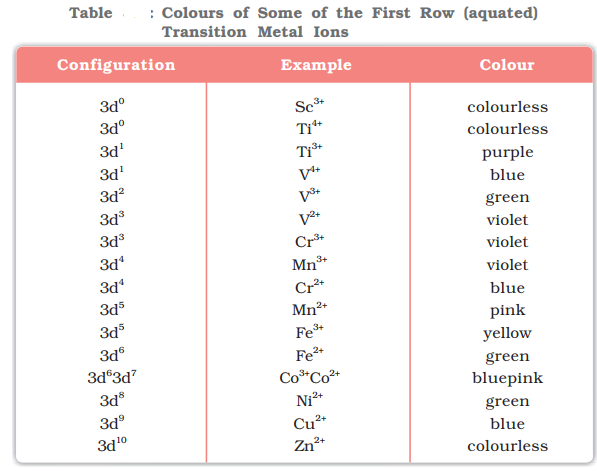
Formation of Coloured Ions:
When an electron from a lower energy d orbital is excited to a higher energy d orbital, the energy of excitation corresponds to the frequency of light absorbed. This frequency generally lies in the visible region. The colour observed corresponds to the complementary colour of the light absorbed.
Catalytic Properties of d-block elements:
Most of transition metals are used as catalysts. This is due to the:
- presence of incomplete or empty d-orbitals,
- large surface area and
- variable oxidation state. For example, Fe, Ni, V2O3, Pt, Mo, Co, etc., are used as catalyst.
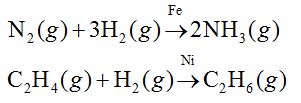
Such complex compounds are not formed by s – and p – block elements.
Explanation: The transition elements form complexes because of the following reasons:
- Comparatively smaller size of their metal ions.
- Their high ionic charges. (Because of (i) and (ii), they have large charge/size ratio)
- Availability of vacant d-orbitals so that these orbitals can accept lone pairs of electrons donated by the ligands.
Interstitial Compounds
The transition metals form a large number of interstitial compounds in which small atoms such as hydrogen, carbon, boron and nitrogen occupy the empty spaces (interstitial sites) in their lattices (Fig.).
They are represented by formulae like TiC, TiH2, Mn4N, Fe3H, Fe3C etc. However, actually they are non-stoichiometric materials, e.g., TiH1.7, VH0.56 etc. and the bonds present in them are neither typically ionic nor covalent. Some of their important characteristics are as follows:
- They are very hard and rigid, e.g., steel which is an interstitial compound of Fe and Cis quite hard. Similarly, some borides are as hard as diamond.
- They have high melting points which are higher than those of the pure metals.
- They show conductivity like that of the pure metal.
- They acquire chemical inertness.

