
Glucose is a monosaccharide’s with molecular formula C6H12O6. It is present in sweet fruits and in honey.
Preparation of Glucose:
#1 Preparation of Glucose From sucrose, C12H22O11:
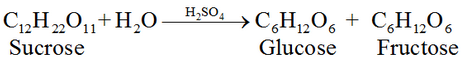
#2 Preparation of Glucose From starch C6H10O5:

Structure of Glucose:
Glucose is an aldohexose and is also known as dextrose. It is the monomer of many of the larger carbohydrates, namely starch, cellulose.
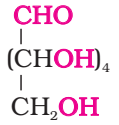
- Its molecular formula was found to be C6H12O6.
- Reaction with HI:

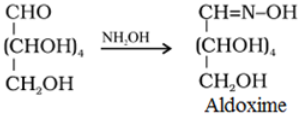
- Reaction with hydroxylamine, NH4OH: Glucose reacts with hydroxylamine to form an oxime and adds a molecule of hydrogen cyanide to give cyanohydrin. These reactions confirm the presence of a carbonyl group (>C = 0) in glucose.
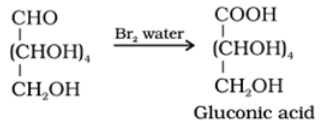
- Reaction with bromine water: Glucose gets oxidised to six carbon carboxylic acid (gluconic acid) on reaction with a mild oxidising agent like bromine water. This indicates that the carbonyl group is present as an aldehydic group.
- Acetylation reaction: Acetylation of glucose with acetic anhydride gives glucose pentaacetate which confirms the presence of five –OH groups. Since it exists as a stable compound, five –OH groups should be attached to different carbon atoms.
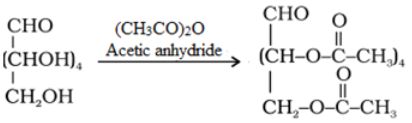
- Oxidation: On oxidation with nitric acid, glucose as well as gluconic acid both yield a dicarboxylic acid, saccharic acid. This indicates the presence of a primary alcoholic (–OH) group in glucose.

Open chain structure(Fisher model):
These two optical isomers differ in configuration around any other C atom other than C1 atom.
In D-Glucose, −OH group on first chiral `C’ from the bottom is on right hand.
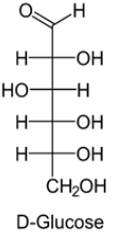
In L-Glucose, −OH group points to the left of chiral carbon.

- Glucose is correctly named as D(+)-glucose. ‘D’ before the name of glucose represents the configuration whereas ‘(+)’ represents dextrorotatory nature of the molecule. It may be remembered that ‘D’ and ‘L’ have no relation with the optical activity of the compound. The meaning of D– and L– notations is given as follows.
- Glyceraldehyde contains one asymmetric carbon atom and exists in two enantiomeric forms as shown below.
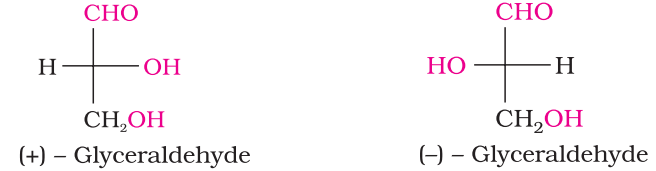
Cyclic Structure of Glucose
Glucose is found to exist in two different crystalline forms which are named as α and β. The α-form of glucose (m.p. 419 K) is obtained by crystallization from concentrated solution of glucose at 303 K while the β-form (m.p. 423 K) is obtained by crystallization from hot and saturated aqueous solution at 371 K.
Both α-D-glucose and β-D-glucose undergo mutarotation in aqueous solution


