
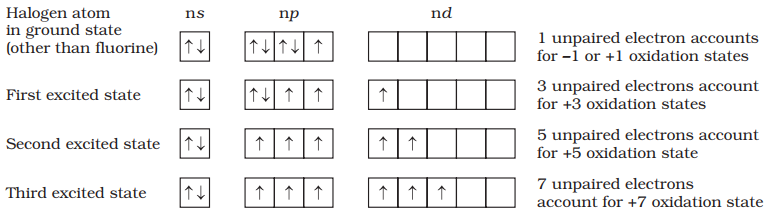
Fluorine, chlorine, bromine, iodine and astatine are members of Group 17. These are collectively known as the halogens (Greek halo means salt and genes born i.e., salt producers). The halogens are highly reactive non-metallic elements.
Electronic Configuration:
All these elements have seven electrons in their outermost shell (ns2 np5) which is one electron short of the next noble gas.
Atomic and Ionic Radii:
The halogens have the smallest atomic radii in their respective periods due to maximum effective nuclear charge. Atomic and ionic radii increase from fluorine to iodine due to increasing number of quantum shells.
Ionisation Enthalpy:
They have little tendency to lose an electron. Thus, they have very high ionisation enthalpy. Due to increase in atomic size, ionisation enthalpy decreases down the group.
Electron Gain Enthalpy:
- Halogen has maximum negative electrons gain enthalpy in the corresponding period. This is due to the fact that the atoms of these elements have only one electron less than stable noble gas configurations.
- Electron gain enthalpy of the elements of the group becomes less negative down the group.
- However, the negative electron gain enthalpy of fluorine is less than that of chlorine.
- It is due to small size of fluorine atom.
- As a result, there are strong interelectronic repulsions in the relatively small 2p orbitals of fluorine and thus, the incoming electron does not experience much attraction
Electronegativity:
They have very high electronegativity. The electronegativity decreases down the group. Fluorine is the most electronegative element in the periodic table
Metallic or non-metallic character:
- Because of very high ionization energy values, all halogens are non-metallic in character.
- The non-metallic character decreases as we go down the group.
- Therefore, the last element, iodine is solid with a metallic lustre and forms positive ions such as I+ and I3+.
Colour:
All the halogens are coloured. The colour of different halogens are given below:
| Halogen | Fluorine | Chlorine | Bromine | Iodine |
|---|---|---|---|---|
| Colour | Light Yellow | Greenish Yellow | Reddish Yellow | Dark Yellow |
Melting and Boiling Point:
The melting and boiling point of halogens increases with increase in atomic number as we go down the group.
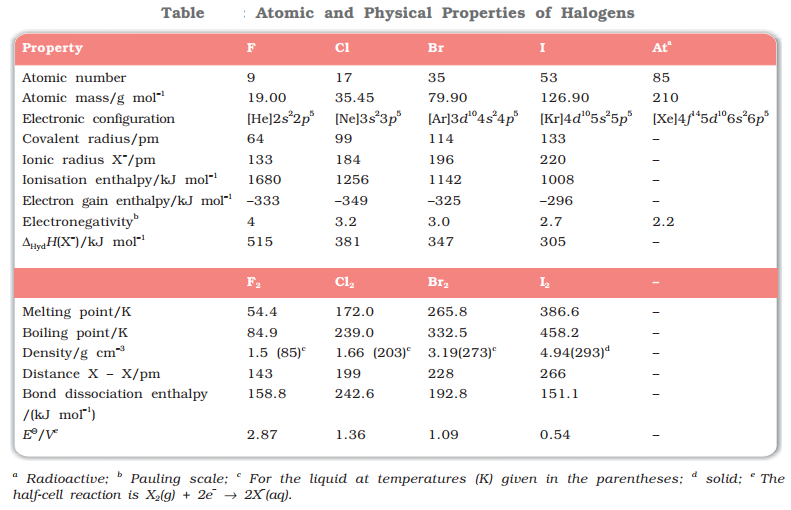
Oxidation State
The most common oxidation state of halogens is −1. Cl, Br, I also show positive oxidation states of +1, +3, + 5, + 7. F does not show positive oxidation states due to non-availability of d-orbitals.
All the halogens are highly reactive. They react with metals and non-metals to form halides. The reactivity of the halogens decreases down the group

