
Hydrogen Bonding
Hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom (F, O or N) of another molecule. It is represented by dotted lines. The chains possess a zig-zag structure.
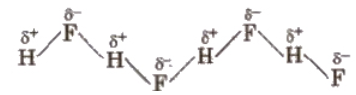
(Hydrogen bond is purely electrostatic and a weak bond. The strength of the strongest hydrogen bond is about 5-10 kcal per mol. The more the electronegativity of atom involved in H-bonding, the more is the bond strength, e.g.,
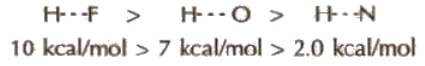
Types of hydrogen bonds are
- Intermolecular H-bonding: H-bonding involving two or more molecules.
- Intramolecular H-bonding: H-bonding within a molecule.
Intermolecular H-bonding:
It is formed between two different molecules of the same or different compounds. For example, H-bond in case of HF molecule, alcohol or water molecules, etc.
Intramolecular H-bonding:
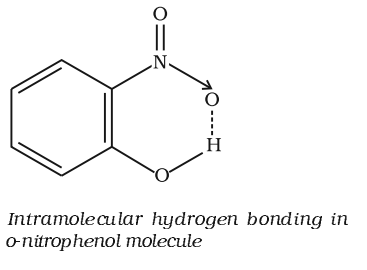
It is formed when hydrogen atom is in between the two highly electronegative (F, O, N) atoms present within the same molecule. For example, in o-nitrophenol the hydrogen is in between the two oxygen atoms.
Strength of bonds
Ionic bond > covalent bond > metallic bond > H-bond

