
Impurity Defects
If molten NaCl containing a little amount of SrCl2 is crystallized, some of the sites of Na+ ions are occupied by Sr2+ (Fig.).
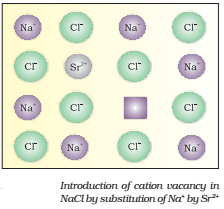
Each Sr2+ replaces two Na+ ions. It occupies the site of one ion and the other site remains vacant. The cationic vacancies thus produced are equal in number to that of Sr2+ ions.
Another similar example is the solid solution of CdCl2 and AgCl.

