
The forces of attraction existing among the molecules of a substance (gaseous, liquid or solid) are called intermolecular forces.
Greater the intermolecular forces, higher is the melting and boiling point.
Attractive intermolecular forces are known as van der Waals’ forces.
The different types of intermolecular forces are briefly explained below:
Dipole-dipole interactions:
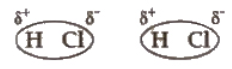
Dipole-dipole forces act between the molecules possessing permanent dipoles. The interaction is stronger than London forces and weaker than ion-ion interaction.
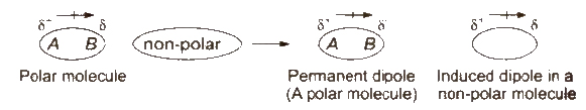
Dipole-induced dipole forces:
Dipole-induced dipole forces act between the polar molecules having permanent dipole and the molecules lacking permanent dipole.
Dispersion forces or London forces:
Dispersion forces or London forces are present among non-polar atoms and molecules, e.g., among the atoms the atoms or chlorine molecules. These are the weakest intermolecular forces. The forces increase with: Increase in number of electrons in molecules, Increase in molecular size
Hydrogen bonding:
When hydrogen atom is attached to highly electronegative element by covalent bond, electrons are shifted towards the more electronegative atom. Thus, a partial positive charge develops on the hydrogen atom. Now, the positively charged hydrogen atom of one molecule may attract the negatively charged atom of some other molecule and the two molecules can be linked together through a weak force of attraction.
![]()
Thermal Energy:
The energy arising due to molecular motion of the body is known as thermal energy. Since motion of the molecules is directly related to kinetic energy is directly proportional to the temperature.

